All Photos(1)
About This Item
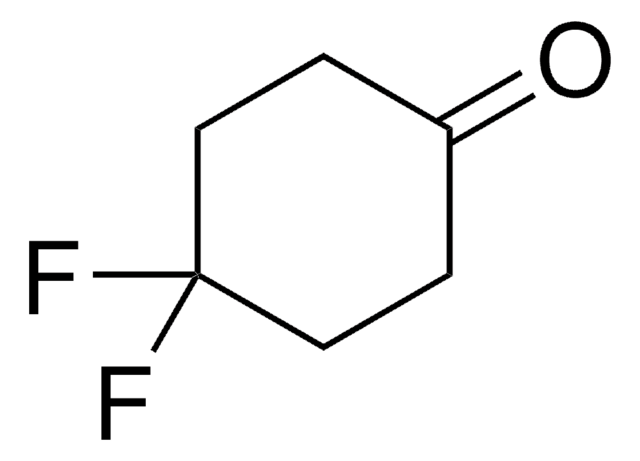
Linear Formula:
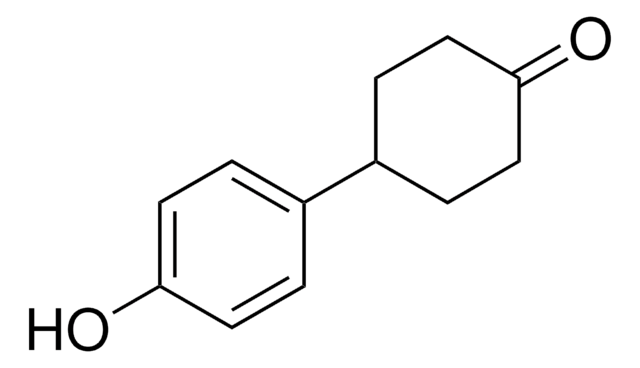
C6H5C6H9(=O)
CAS Number:
Molecular Weight:
174.24
Beilstein/REAXYS Number:
2045904
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥98%
form
solid
mp
73-77 °C (lit.)
SMILES string
O=C1CCC(CC1)c2ccccc2
InChI
1S/C12H14O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-5,11H,6-9H2
InChI key
YKAYMASDSHFOGI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Phenylcyclohexanone undergoes Ruthenium-catalyzed reaction with tributylamine to yield 2-butyl-4-phenylcyclohexanone and 2,6-dibutyl-4-phenylcyclohexanone. Wittig reaction of 4-phenylcyclohexanone with (carbethoxymethylene)triphenylphosphorane under microwave irradiation has been investigated.
Application
4-Phenylcyclohexanone was used in the preparation of new cardo diamine monomer, 1,1-bis[4-(4-aminophenoxy)phenyl]-4-phenylcyclohexane bearing a 4-phenylcyclohexylidene unit.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
212.0 °F - closed cup
flash_point_c
100 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and Characterization of New Cardo Polyamides and Polyimides bearing a 4-Phenylcyclohexylidene Unit.
Liaw D-J, et al.
Macromolecular Chemistry and Physics, 202(6), 807-813 (2001)
Ruthenium-Catalyzed Regioselective alpha-Alkylation of Ketones: The First Alkyl-Group Transfer from Trialkylamines to the alpha-C Atom of Ketones This work was supported by the Basic Research Program of the Korea Science and Engineering Foundation (2000-2-12200-001-3). C.S.C. gratefully acknowledges a Post-Doctoral Fellowship of Kyungpook National University (2000).
Chan Sik Cho et al.
Angewandte Chemie (International ed. in English), 40(5), 958-960 (2001-03-10)
Highly regioselective Wittig reactions of cyclic ketones with a stabilized phosphorus ylide under controlled microwave heating.
Wu J, et al.
Tetrahedron Letters, 45(22), 4401-4404 (2004)
Alejandro Gran-Scheuch et al.
Frontiers in microbiology, 9, 1609-1609 (2018-08-04)
Actinobacteria are an important source of commercial (bio)compounds for the biotechnological and pharmaceutical industry. They have also been successfully exploited in the search of novel biocatalysts. We set out to explore a recently identified actinomycete, Streptomyces leeuwenhoekii C34, isolated from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service