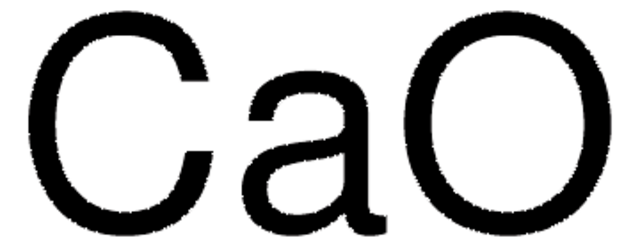208159
Calcium oxide
ReagentPlus®, 99.9% trace metals basis
Synonym(s):
Lime, Quicklime
About This Item
Recommended Products
Quality Level
product line
ReagentPlus®
assay
99.9% trace metals basis
form
powder
reaction suitability
reagent type: catalyst
core: calcium
loss
10.0% loss on ignition, 1000°C (absorbed H2O and CO2)
bp
2850 °C (lit.)
density
3.3 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
O=[Ca]
InChI
1S/Ca.O
InChI key
ODINCKMPIJJUCX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives: Discusses the use of calcium oxide derived from natural sources like limestone, seashells, and eggshells as an effective catalyst in biodiesel production (Marinković et al., 2016).
Legal Information
signalword
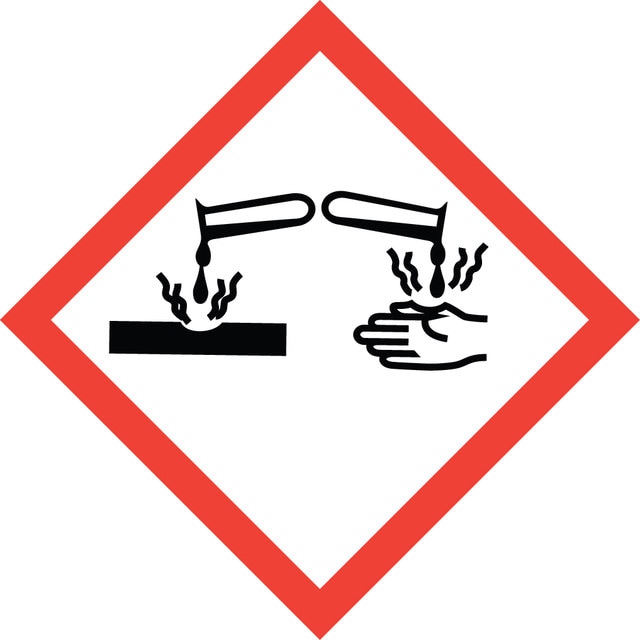
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Thermoelectric Performance of Perovskite-type Oxide Materials
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service