378151
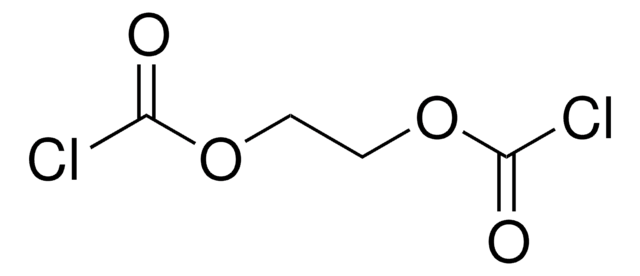
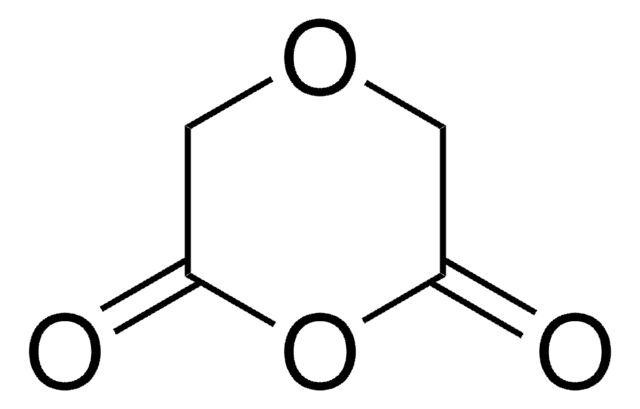
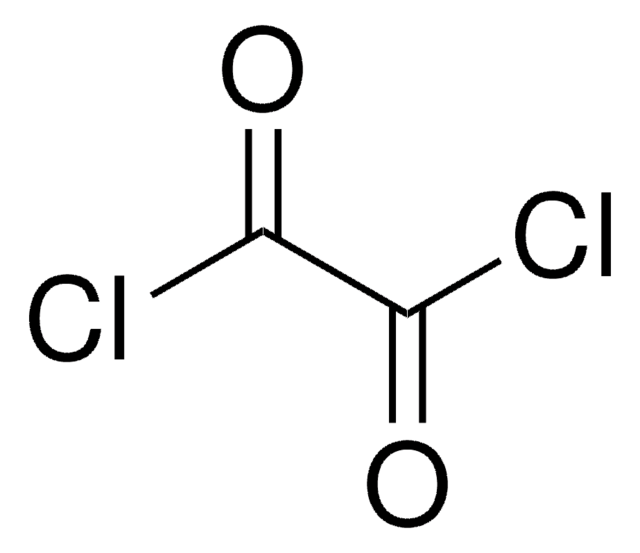
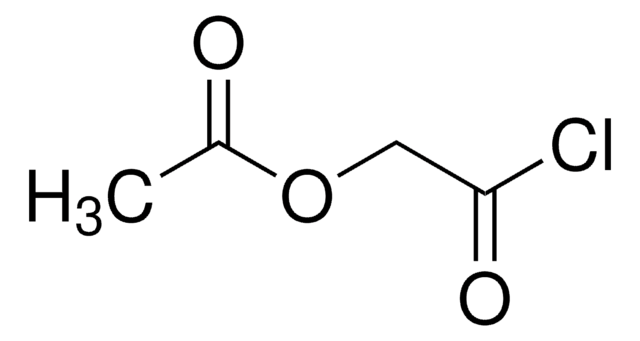
Diglycolyl chloride
95%
Synonym(s):
2,2′-Oxydiacetyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
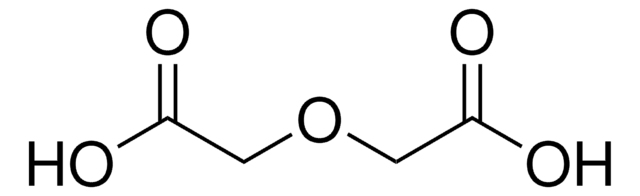
O(CH2COCl)2
CAS Number:
Molecular Weight:
170.98
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
form
liquid
refractive index
n20/D 1.473 (lit.)
bp
84-87 °C/2 mmHg (lit.)
density
1.439 g/mL at 25 °C (lit.)
SMILES string
ClC(=O)COCC(Cl)=O
InChI
1S/C4H4Cl2O3/c5-3(7)1-9-2-4(6)8/h1-2H2
InChI key
GTZXSBQCNBNWPK-UHFFFAOYSA-N
Related Categories
General description
Diglycolyl chloride (2,2′-Oxydiacetyl chloride) is an acid halide.
Application
Diglycolyl chloride is suitable for use in the synthesis of ply(ether ester). It may be used in the synthesis of:
- chiral diphenyl substituted polyether-diester compounds
- morpholine dione analog (IMDNQ)
- salicylic acid (SA)- based diacids
Diglycolyl chloride may be used in the synthesis of the following compounds:
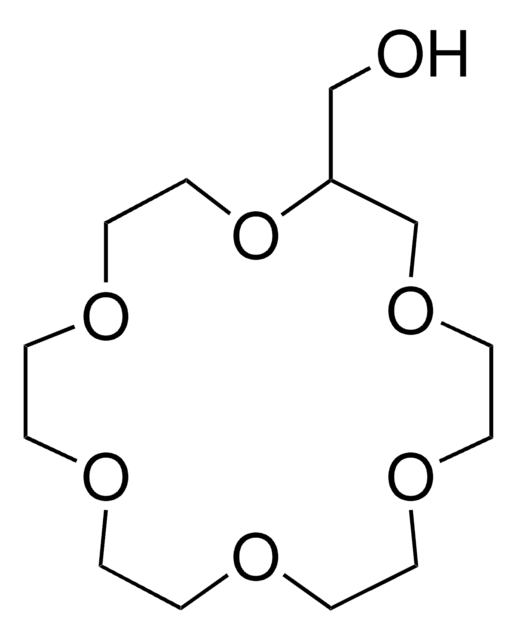
- diazadibenzo-18-crown-6 diamide
- diazadi(tert-butylbenzo)-18-crown-6 diamide
- surfen derivative
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
wgk_germany
WGK 3
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of chiral diphenyl substituted polyether-diester compounds.
Bradshaw JS, et al.
The Journal of Organic Chemistry, 47(7), 1229-1232 (1982)
Ashley L Carbone et al.
Macromolecular rapid communications, 30(12), 1021-1021 (2010-02-18)
Fast-degrading, salicylate-based poly(anhydride-esters) were designed to degrade and release the active component, salicylic acid (SA), within 1 week. The polymer degradation was enhanced by using shorter or oxygen-containing aliphatic chains. A copolymer of diglycolic acid was also made with a
Synthesis and CO2 Solubility Studies of Poly (ether carbonate) s and Poly (ether ester) s Produced by Step Growth Polymerization.
Tan B, et al.
Macromolecules, 38(5), 1691-1698 (2005)
J C Aguilar et al.
Talanta, 54(6), 1195-1204 (2008-10-31)
The ligands 4,7-diaza-2,3,8,9-dibenzo-15-crown-5 (L1), 4,10-diaza-2,3,11,12-dibenzo-18-crown-6 (L2), 4,10-diaza-2,3,11,12-di(4'-tert-butylbenzo)-18-crown-6 (L3) and N,N-di(methylenecarboxyethoxy) 4,10-diaza-2,3,11,12-dibenzo-18-crown-6 (L4) have been prepared. Partition coefficients and acid dissociation constants for these four diazadibenzocrown ether compounds were determined in water-chloroform. Their effectiveness was assessed in solvent extraction of Pb(2+)
Small molecule antagonists of cell-surface heparan sulfate and heparin-protein interactions.
Weiss RJ, et al.
Chemical Science, 6(10), 5984-5993 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service