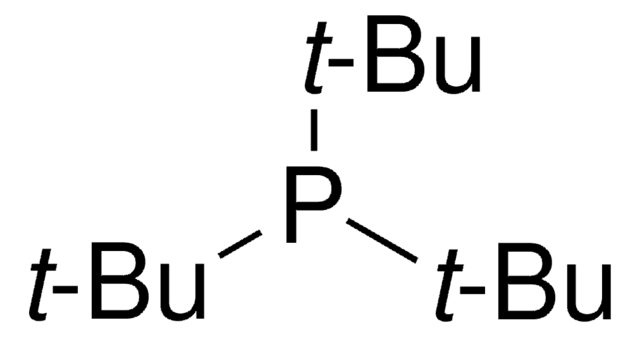379875
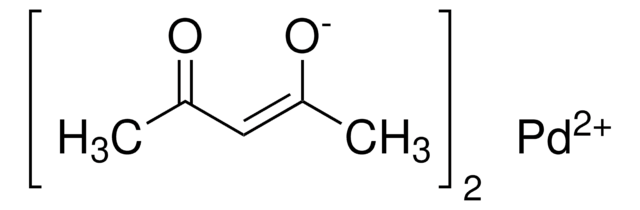
[Pd(OAc)2]3
99.98% trace metals basis
Synonym(s):
Palladium(II) acetate, Pd(OAc)2
About This Item
Recommended Products
Quality Level
assay
99.98% trace metals basis
form
powder
reaction suitability
core: aluminum
reagent type: catalyst
mp
216.3-223.7 °C (dec.)
SMILES string
CC(O[Pd]OC(C)=O)=O
InChI
1S/2C2H4O2.Pd/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
YJVFFLUZDVXJQI-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
signalword
Danger
hcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Sens. 1A
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
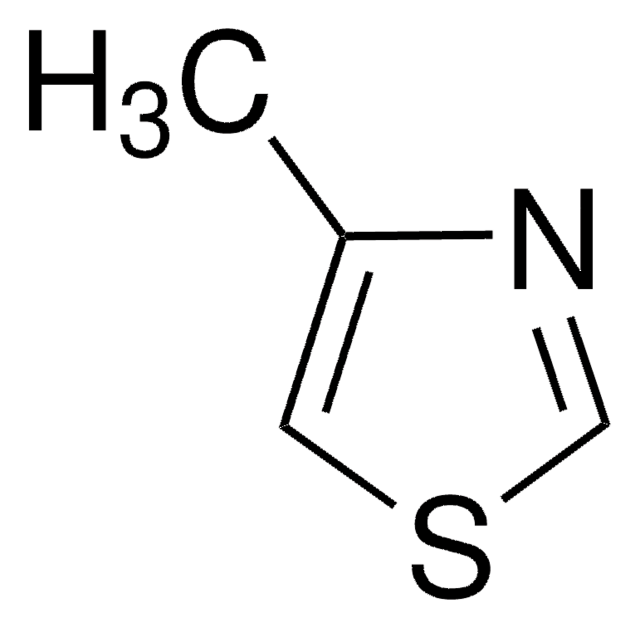
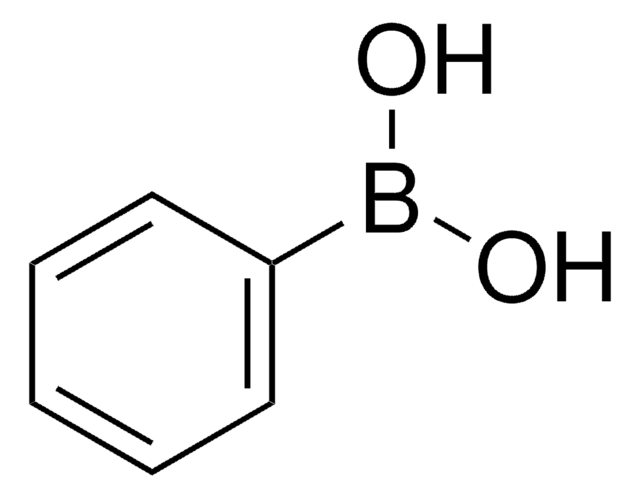
A variety of transition-metal catalysts for the Suzuki coupling reaction are now available in our catalog. The majority of these catalysts are palladium- and nickelbased, typically utilizing phosphine-derived ligands.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)