All Photos(1)
About This Item
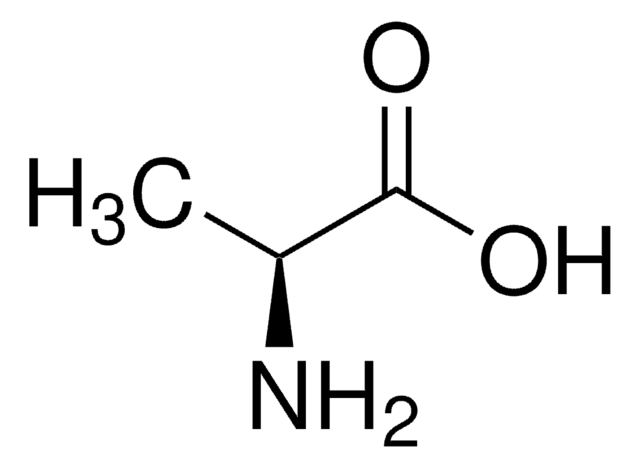
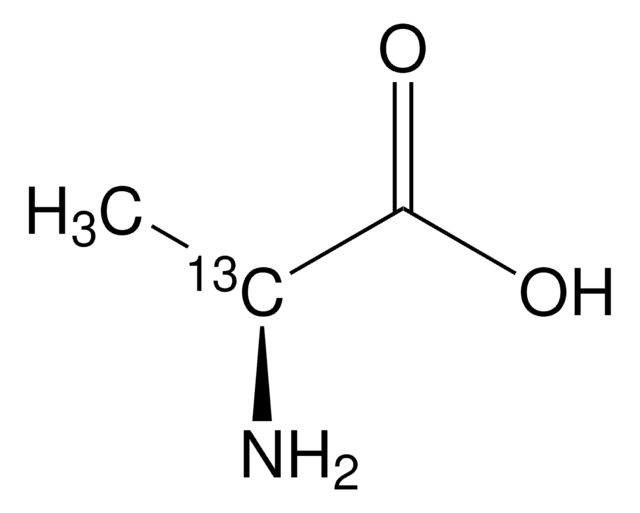
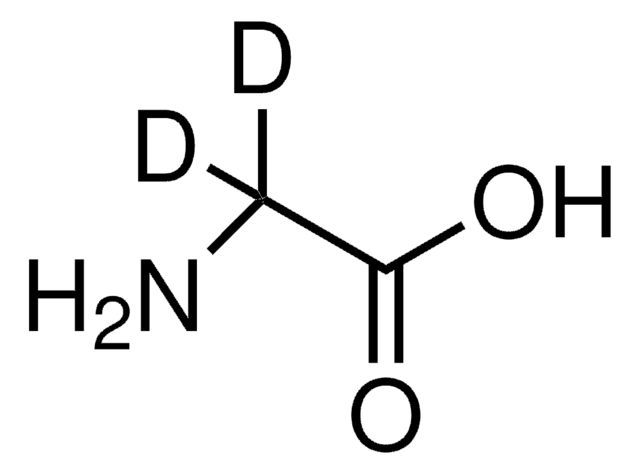
Linear Formula:
12CH312CH(NH2)12CO2H
CAS Number:
Molecular Weight:
89.06
Beilstein/REAXYS Number:
1720248
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
Recommended Products
description
13C-depleted
Quality Level
isotopic purity
99.9 atom % 12C
optical activity
[α]25/D +14.5°, c = 2 in 1 M HCl
availability
available only in Japan
mp
314.5 °C (dec.) (lit.)
mass shift
depleted
SMILES string
[12CH3][12C@H](N)[12C](O)=O
InChI
1S/C3H7NO2/c1-2(4)3(5)6/h2H,4H2,1H3,(H,5,6)/t2-/m0/s1/i1+0,2+0,3+0
InChI key
QNAYBMKLOCPYGJ-BWIFZRRMSA-N
Related Categories
Biochem/physiol Actions
L-Alanine is a nonessential amino acid, which is highly concentrated in muscle. It is a key player in the Glucose-Alanine cycle, which enables the removal of pyruvate and glutamate from muscle to the liver. Once in the liver, glucose is regenerated from pyruvate and returned to the muscle while glutamate ultimately participates in the urea cycle to form urea. The Glucose-Alanine cycle aides to conserve ATP in muscle for muscle contraction, while the energy burden of gluconeogenesis is imposed upon the liver. Alanine inhibits pyruvate kinase to regulate gluconeogenesis and glycolysis in order to maintain glucose homeostasis during starvation. Alanine prevents hepatic autophagy. Alanine formation is a result of transamination of glutamate and pyruvate.
Packaging
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis.
Garber A J, et al.
The Journal of Biological Chemistry, 251(3), 836-843 (1976)
Muscle alanine synthesis and hepatic gluconeogenesis
Snell K
Biochemical Society Transactions, 8(2), 205-213 (1980)
Mom Das et al.
Nucleic acids research, 42(6), 3943-3953 (2013-12-29)
Errors in protein synthesis due to mispairing of amino acids with tRNAs jeopardize cell viability. Several checkpoints to prevent formation of Ala- and Cys-tRNA(Pro) have been described, including the Ala-specific editing domain (INS) of most bacterial prolyl-tRNA synthetases (ProRSs) and
Lillian L Siu et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 31(19), 2477-2484 (2013-05-22)
The antiepidermal growth factor receptor monoclonal antibody cetuximab has improved survival in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal cancer. The addition of brivanib, a tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor and fibroblast growth factor receptor, to
Rachelle S Doody et al.
The New England journal of medicine, 369(4), 341-350 (2013-07-26)
Alzheimer's disease is characterized by the presence of cortical amyloid-beta (Aβ) protein plaques, which result from the sequential action of β-secretase and γ-secretase on amyloid precursor protein. Semagacestat is a small-molecule γ-secretase inhibitor that was developed as a potential treatment
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service