551473
QuadraPure® AK
50-90 mesh, extent of labeling: 2.0-3.0 mmol/g loading, 1 % cross-linked with divinylbenzene
Synonym(s):
2,4-Butanedione resin, Activated ketone, polymer-bound
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
product line
QuadraPure®
Quality Level
crosslinking
1 % cross-linked with divinylbenzene
reaction suitability
reaction type: solution phase peptide synthesis
reactivity: nucleophile reactive
extent of labeling
2.0-3.0 mmol/g loading
particle size
50-90 mesh
Application
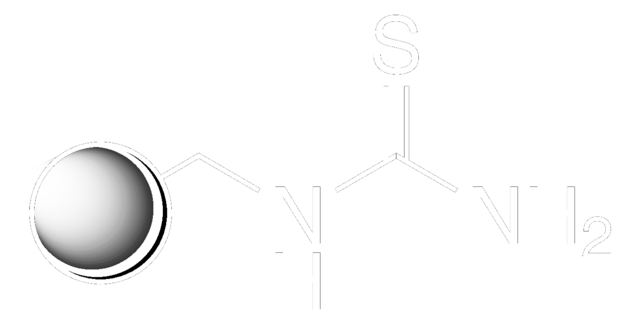
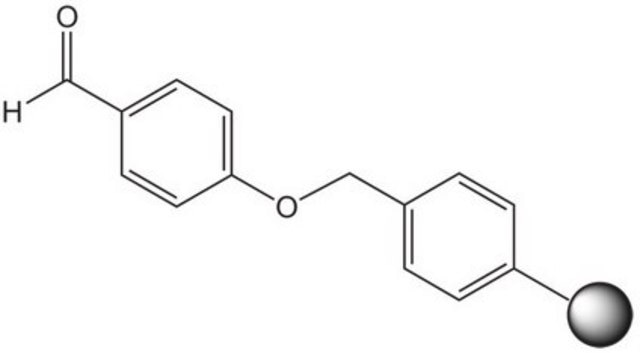
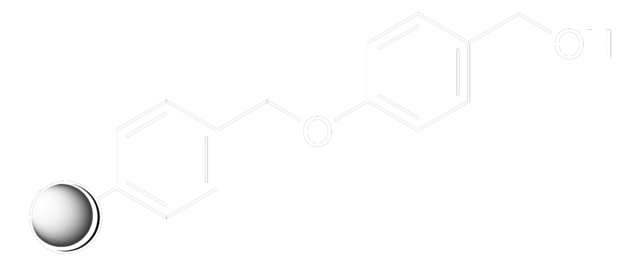
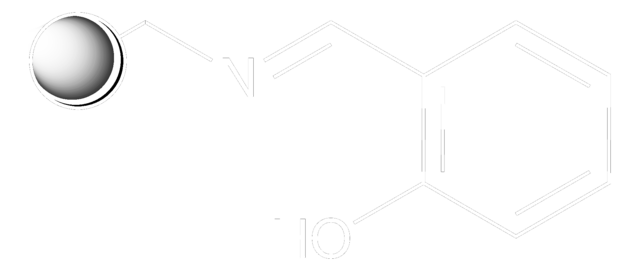
QuadraPure® AK is a resin which is generally used as a support for heterogeneous catalysis. It can be used as a hydroxylamine scavenger.
Scavenger for Hydrazines and Primary Amines
Legal Information
QuadraPure is a registered trademark of Johnson Matthey Finland Oy
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yu, Z. et al.
Tetrahedron Letters, 41, 8963-8963 (2000)
Quadrapure-supported palladium nanocatalysts for microwave-promoted Suzuki cross-coupling reaction under aerobic condition
Liew KH, et al.
TheScientificWorldJournal, 2014 (2014)
A Polymer-Bound 1,3-Diketone: A Highly Efficient Scavenger for Hydrazines, and Primary Amines
Uwe Schon, Josef Messinger, Nuria Merayo, Grzegorz Juszkiewicz, Andreas Kirschning
Synlett, 7, 983-983 (2003)
Flow synthesis of tricyclic spiropiperidines as building blocks for the histrionicotoxin family of alkaloids
Brasholz M, et al.
Tetrahedron, 66(33), 6445-6449 (2010)
QuadraPure Cartridges for Removal of Trace Metal from Reaction Mixtures in Flow
A. Hinchcliffe, et al.
Organic Process Research & Development, 11, 477-481 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service