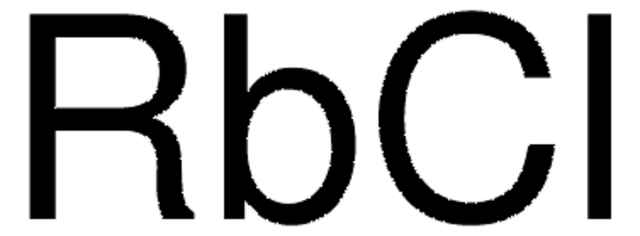All Photos(1)
About This Item
CAS Number:
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
Quality Level
concentration
~13% Mg
loss
≤10% loss on drying
SMILES string
O.[Mg++].[Mg++].O[Si]([O-])=O.O[Si]([O-])=O.[O-][Si]([O-])=O
InChI
1S/2Mg.2HO3Si.O3Si.H2O/c;;3*1-4(2)3;/h;;2*1H;;1H2/q2*+2;2*-1;-2;
InChI key
TUKQLEWOUPCTOS-UHFFFAOYSA-N
Related Categories
General description
Sepiolite is a hydrated magnesium silicate that belongs to the class of sepiolite-palygorskite. Its properties include high absorption capacity and good rheological behavior.
Application
Sepiolites can be used as absorbents for potential applications in catalytic systems and wastewater treatment.
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite
Alkan M, et al.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 101(3), 388-396 (2007)
Experimental and Atomic Modeling of the Adsorption of Acid Azo Dye 57 to Sepiolite
Karatacs D, et al.
Clays and Clay Minerals, 66(5), 426-437 (2018)
Thermal and morphological characterisation of organically modified sepiolite
Tartaglione G, et al.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 107(1-2), 161-168 (2008)
Xuefeng Liang et al.
Chemosphere, 90(2), 548-555 (2012-09-22)
Mercapto functionalized sepiolite (MSEP) was prepared by nanotexturization method and applied for the sorption of Pb(2+) from aqueous solution. These samples before and after sorption were characterized through XRD, FT-IR, (29)Si and (13)C CP/MAS NMR and XPS. The sorption behaviors
R Portela et al.
Environmental science & technology, 46(9), 5040-5048 (2012-03-27)
The photocatalytic efficiency of TiO(2)-SiMgO(x) plates to oxidize H(2)S was first evaluated in a flat laboratory reactor with 50 mL min(-1) synthetic air containing 100 ppm H(2)S in the presence of humidity. The use of the photocatalyst-adsorbent hybrid material enhanced
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service