76050
Palladium(II) chloride
anhydrous, 60% Pd basis
Synonym(s):
Dichloropalladium, Palladium dichloride, Palladous chloride
About This Item
Recommended Products
Quality Level
form
solid
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
concentration
60% Pd
mp
678-680 °C (lit.)
density
4 g/mL at 25 °C (lit.)
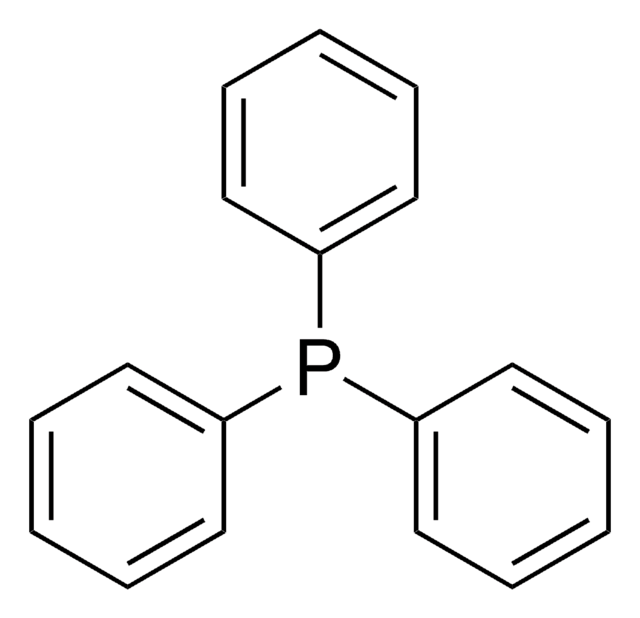
SMILES string
Cl[Pd]Cl
InChI
1S/2ClH.Pd/h2*1H;/q;;+2/p-2
InChI key
PIBWKRNGBLPSSY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Used in the synthesis of semiconducting metal-containing polymers in which the polypyrrole backbone has a conformational energy minimum and is nearly planar.
- Cross-coupling reactions between terminal alkynes and aryl iodides or bromides under modified Sonogashira-Cassar-Heck conditions.
- Carbonylation of organic tellurides with carbon monoxide to form corresponding methyl carboxylates.
- Isomerization of allylic ester in acetic acid.
- Carbonylation of amines to form isocyanates.
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Met. Corr. 1 - Skin Sens. 1
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service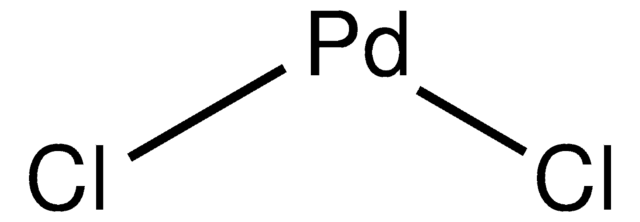
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)