851590
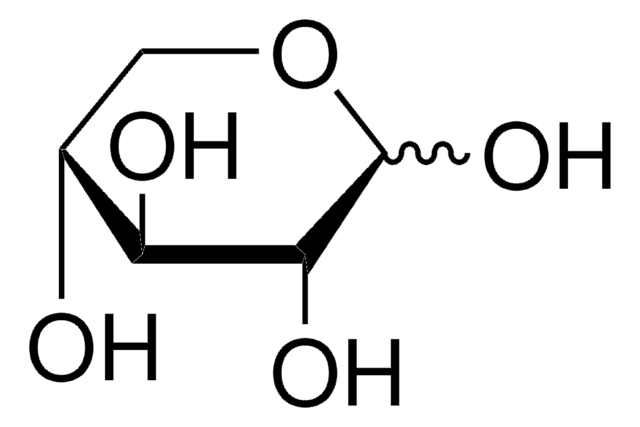
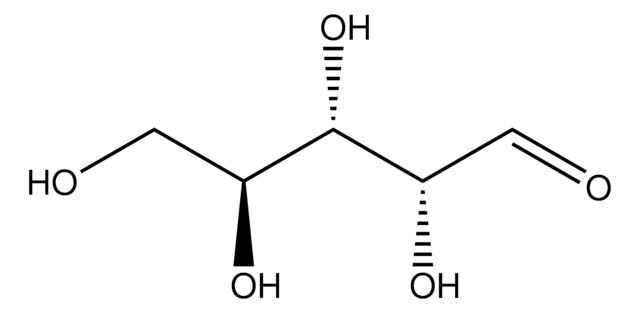
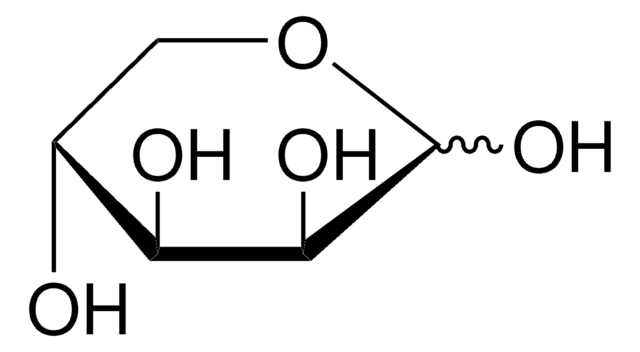
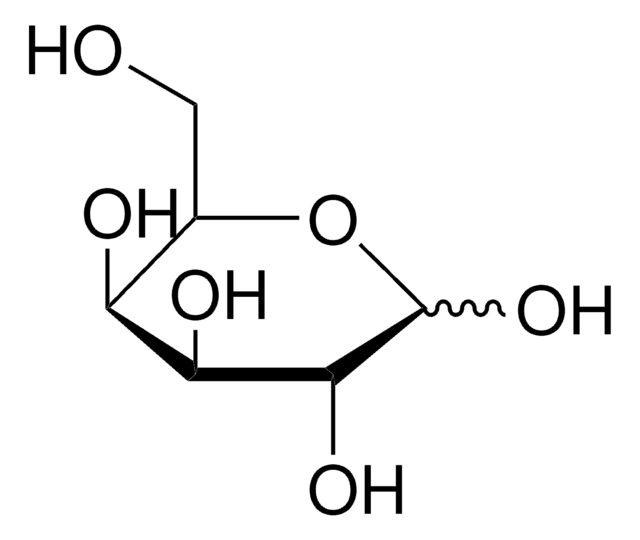
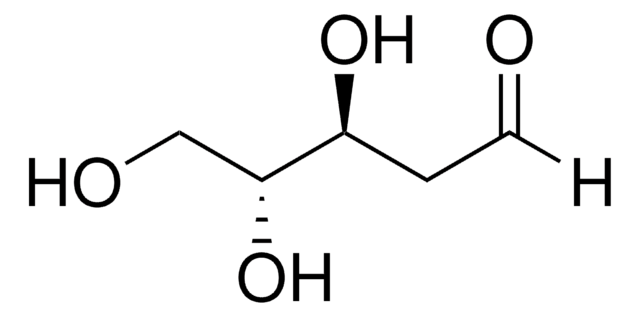
L-(−)-Xylose, mixture of anomers
≥99%
Synonym(s):
L-Xylose
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H10O5
CAS Number:
Molecular Weight:
150.13
Beilstein/REAXYS Number:
1723080
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥99%
form
powder
optical activity
[α]24/D −18.7°, c = 4 in H2O
mp
150-152 °C (lit.)
SMILES string
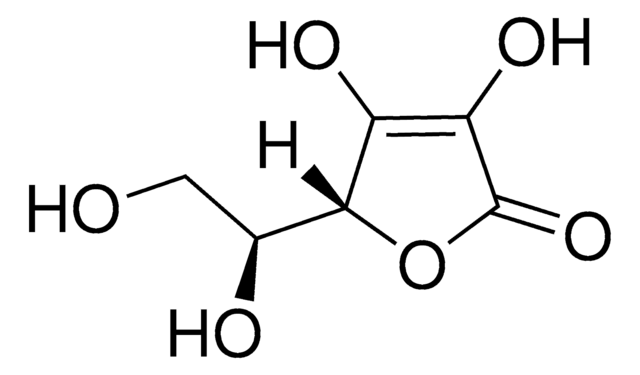
O[C@H]1COC(O)[C@@H](O)[C@@H]1O
InChI
1S/C5H10O5/c6-1-3(8)5(10)4(9)2-7/h1,3-5,7-10H,2H2/t3-,4+,5+/m1/s1
InChI key
PYMYPHUHKUWMLA-WISUUJSJSA-N
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yang Liu et al.
Antonie van Leeuwenhoek, 109(2), 207-213 (2015-11-22)
Four Gram-stain positive bacterial strains, designated as 4R1(T), 4R9, 4L13 and 4L18, isolated from seeds of hybrid maize (Zea mays L., Jingke 968), were investigated using a polyphasic taxonomic approach. The cells were found to be facultatively aerobic, motile, spore-forming
Misun Lee et al.
Biotechnology for biofuels, 13, 5-5 (2020-01-16)
Efficient bioethanol production from hemicellulose feedstocks by Saccharomyces cerevisiae requires xylose utilization. Whereas S. cerevisiae does not metabolize xylose, engineered strains that express xylose isomerase can metabolize xylose by converting it to xylulose. For this, the type II xylose isomerase
Kyle V Probst et al.
Biotechnology progress, 33(4), 1096-1103 (2017-04-04)
Cyclopentyl methyl ether (CPME) was evaluated for extracting oil or triacylglycerol (TAG) from wet cells of the oleaginous yeast Lipomyces starkeyi. CPME is a greener alternative to chloroform as a potential solvent for oil recovery. A monophasic system of CPME
Fayin Zhu et al.
Journal of industrial microbiology & biotechnology, 47(2), 223-232 (2020-01-29)
It is of great economic interest to produce succinate from low-grade carbon sources, e.g., lignocellulosic biomass hydrolysate, which mainly contains glucose and xylose. Inactivation of the glucose uptake system PtsG was evaluated for succinate production from xylose-rich feedstocks. Strains with
Julian Quehenberger et al.
International journal of molecular sciences, 20(1) (2019-01-10)
While in search of an enzyme for the conversion of xylose to xylitol at elevated temperatures, a xylose reductase (XR) gene was identified in the genome of the thermophilic fungus Chaetomium thermophilum. The gene was heterologously expressed in Escherichia coli
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service