907723
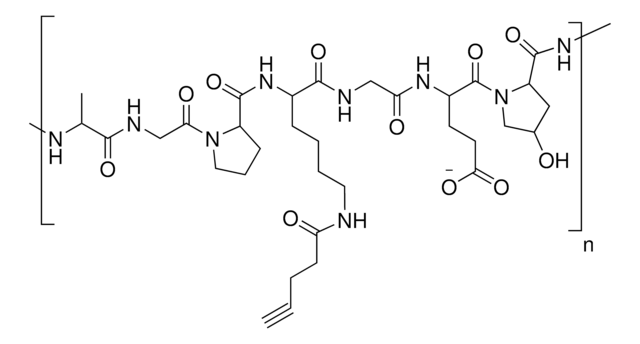
Azide functionalized gelatin
degree of substitution >80%
Synonym(s):
Azide functionlized gelatin, Azide-modified gelatin, Clickable gelatin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352125
NACRES:
NA.23
Recommended Products
description
Degree of substitution: greater than 80% by TNBS method
NMR: Conforms to structure
form
powder
color
white to pale yellow
storage temp.
−20°C
Related Categories
General description
Due to its biodegradablity and biocompatibility, gelatin is routinely used in hydrogels for biomedical applications such as drug delivery, tissue engineering, and 3D bioprinting. Gelatin-based hydrogels are synthesized by the crosslinking of functionalized gelatins. Depending on the identity of the functional groups, several different processes can be used to synthesize crosslinked gelatin hydrogels, including radical-based (either thermal or photochemical) and click chemistry methods. Azide-functionalized gelatin can be used in the synthesis of hydrogels using click chemistry with alkyne-containing substrates.
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Gelatin hydrogels via thiol-ene chemistry
Russo L, et al.
Monatshefte fur Chemie / Chemical Monthly, 147, 587-592 (2016)
Vinh X Truong et al.
Biomacromolecules, 16(7), 2246-2253 (2015-06-10)
In this study, we present a method for the fabrication of in situ forming gelatin and poly(ethylene glycol)-based hydrogels utilizing bioorthogonal, strain-promoted alkyne-azide cycloaddition as the cross-linking reaction. By incorporating nitrobenzyl moieties within the network structure, these hydrogels can be
Thiol-yne `click?/coupling chemistry and recent applications in polymer and materials synthesis and modification.
Andrew B. Lowe
Polymer, 55 (2014)
Sandeep T Koshy et al.
Advanced healthcare materials, 5(5), 541-547 (2016-01-26)
Injectable gelatin hydrogels formed with bioorthogonal click chemistry (ClickGel) are cell-responsive ECM mimics for in vitro and in vivo biomaterials applications. Gelatin polymers with pendant norbornene (GelN) or tetrazine (GelT) groups can quickly and spontaneously crosslink upon mixing, allowing for
Masato Tamura et al.
Scientific reports, 5, 15060-15060 (2015-10-10)
This paper describes the generation of "click-crosslinkable" and "photodegaradable" gelatin hydrogels from the reaction between dibenzocycloctyl-terminated photoclevable tetra-arm polyethylene glycol and azide-modified gelatin. The hydrogels were formed in 30 min through the click-crosslinking reaction. The micropatterned features in the hydrogels were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service