ALD00564
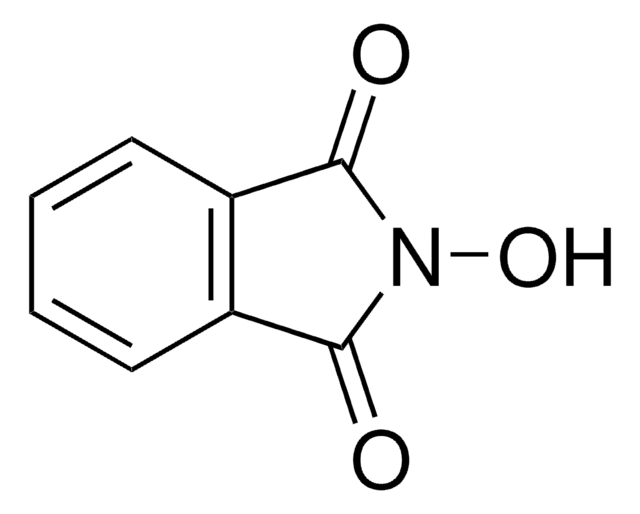
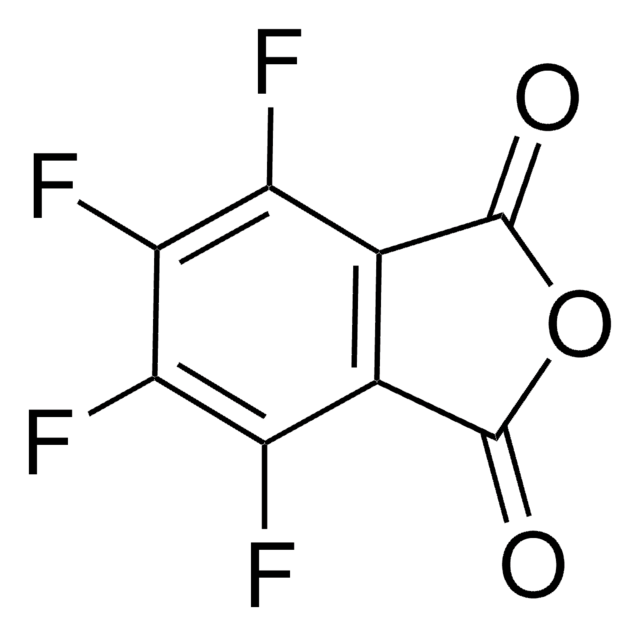
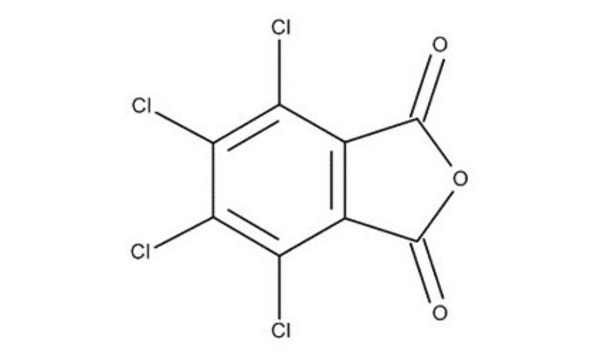
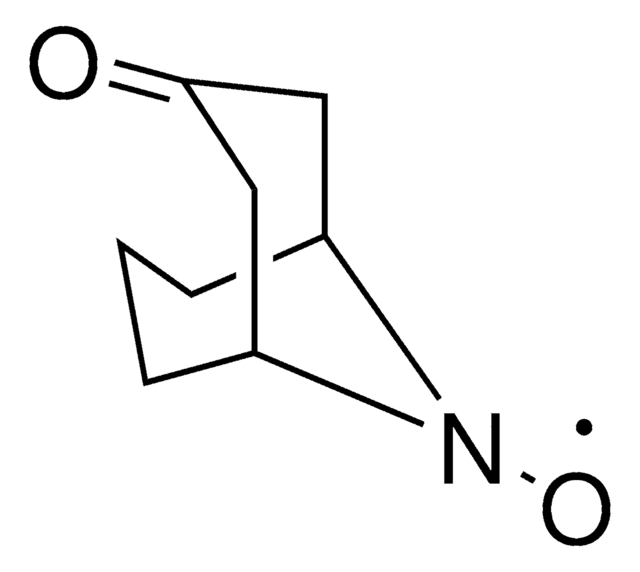
N-Hydroxytetrachlorophthalimide
Synonym(s):
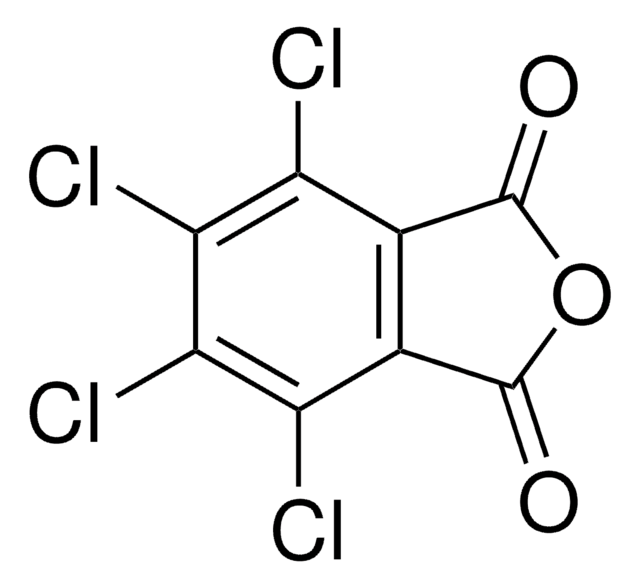
4,5,6,7-Tetrachloro-2-hydroxy-1H-isoindole-1,3(2H)-dione, Tetrachloro-N-hydroxyphthalimide
About This Item
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: oxidant
SMILES string
O=C1N(O)C(C2=C(Cl)C(Cl)=C(Cl)C(Cl)=C21)=O
InChI
1S/C8HCl4NO3/c9-3-1-2(4(10)6(12)5(3)11)8(15)13(16)7(1)14/h16H
InChI key
UTRBHXSKVVPTLY-UHFFFAOYSA-N
Related Categories
General description
Application
Other Notes
Scalable and sustainable electrochemical allylic C–H oxidation
A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents
Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids
Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Phil Baran and coworkers have developed a new reagent, N-Hydroxytetrachlorophthalimide (ALD00564), which provides a cheap, scalable, and safe synthetic alternative to highly used transformations like allylic oxidations, as well as Negishi and Suzuki–Miyaura type cross-coupling reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service