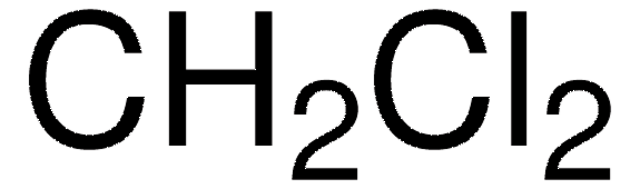About This Item
Recommended Products
vapor density
3 (20 °C, vs air)
Quality Level
vapor pressure
73 mmHg ( 20 °C)
assay
100% (GC)
form
liquid
autoignition temp.
801 °F
expl. lim.
2.2-11.5 %, 38 °F
refractive index
n20/D 1.3720 (lit.)
bp
76.5-77.5 °C (lit.)
mp
−84 °C (lit.)
solubility
water: soluble
density
0.902 g/mL at 25 °C (lit.)
format
neat
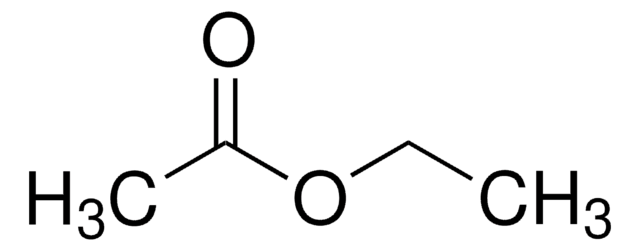
SMILES string
CCOC(C)=O
InChI
1S/C4H8O2/c1-3-6-4(2)5/h3H2,1-2H3
InChI key
XEKOWRVHYACXOJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a solvent for the isolation of Rose hip (Rosa canina L., Rosaceae) powder, via sonication.
- As a solvent for the abstraction of volatile thiols from wine for their quantitative estimation by gas chromatography/mass spectrometry (GC-MS).
- Preparation of thin films of TiO2 (titanium dioxide) on glass.
- As an extraction medium in the multi-residue analysis of pesticide residues in fruit and vegetables.
- Acetylaton of primary amines to form amides in the presence of dimethyltin(IV) acetic acid distannoxane.
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
target_organs
Central nervous system
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
24.8 °F
flash_point_c
-4 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service