W304409
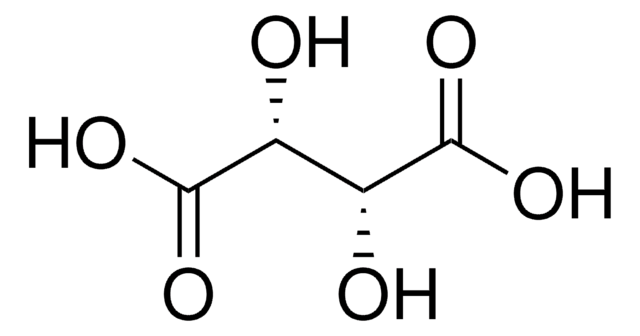
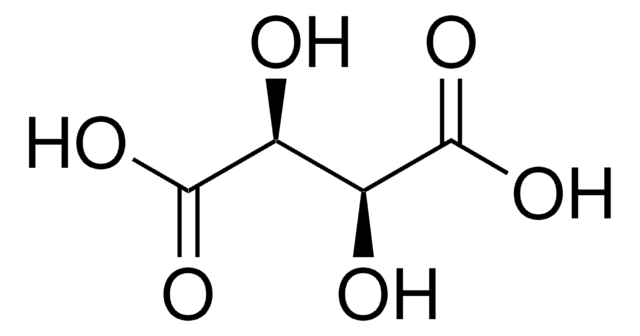
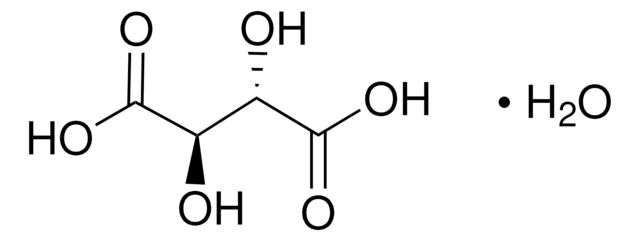
L-(+)-Tartaric acid
≥99.7%, FCC, FG
Synonym(s):
(2R,3R)-(+)-Tartaric acid, L-Threaric acid
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Halal
agency
meets purity specifications of JECFA
reg. compliance
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 163.110
FDA 21 CFR 163.111
FDA 21 CFR 163.112
FDA 21 CFR 184.1099
vapor density
5.18 (vs air)
assay
≥99.7%
form
crystalline powder
optical activity
[α]20/D +12.5°, c = 20 in H2O
autoignition temp.
797 °F
mp
170-172 °C (lit.)
solubility
water: soluble 150 g/L at 25 °C
cation traces
As: ≤3 ppm
Cd: ≤1 ppm
Hg: ≤1 ppm
heavy metals (as Pb): ≤2 ppm
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
organoleptic
odorless
SMILES string
O[C@H]([C@@H](O)C(O)=O)C(O)=O
InChI
1S/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/t1-,2-/m1/s1
InChI key
FEWJPZIEWOKRBE-JCYAYHJZSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Terahertz-spectroscopy for non-destructive determination of crystallinity of L-tartaric acid in smartFilms and tablets made from paper.: This study leverages terahertz spectroscopy to assess the crystallinity of L-(+)-tartaric acid in innovative pharmaceutical applications, enhancing non-destructive testing methods for quality control (Ornik et al., 2020 May). Link to the article.
- Enhanced pulmonary absorption of poorly soluble itraconazole by micronized cocrystal dry powder formulations.: Research shows the use of L-(+)-tartaric acid in cocrystal formulations with itraconazole to improve its pulmonary absorption, demonstrating a significant advancement in drug delivery technologies (Karashima et al., 2017 Jun). Link to the article.
- Physicochemical Evaluation and Developability Assessment of Co-amorphouses of Low Soluble Drugs and Comparison to the Co-crystals.: This article discusses the role of L-(+)-tartaric acid in enhancing the solubility and bioavailability of pharmaceuticals through co-amorphous systems, offering a critical insight into drug formulation strategies (Yamamoto et al., 2016 Dec). Link to the article.
- Functionalized polycarbonate derived from tartaric acid: enzymatic ring-opening polymerization of a seven-membered cyclic carbonate.: This research explores the synthesis of biodegradable polymers from L-(+)-tartaric acid, emphasizing its utility in developing environmentally friendly materials (Wu et al., 2008 Oct). Link to the article.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1
wgk_germany
WGK 1
flash_point_f
302.0 °F
flash_point_c
150 °C
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service