Z-004
Zaleplon solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
SMILES string
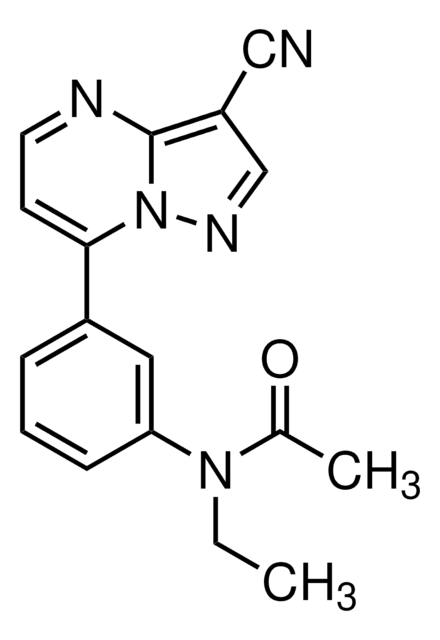
CCN(C(C)=O)c1cccc(c1)-c2ccnc3c(cnn23)C#N
InChI
1S/C17H15N5O/c1-3-21(12(2)23)15-6-4-5-13(9-15)16-7-8-19-17-14(10-18)11-20-22(16)17/h4-9,11H,3H2,1-2H3
InChI key
HUNXMJYCHXQEGX-UHFFFAOYSA-N
Gene Information
human ... GABRA1(2554) , GABRB1(2560) , GABRG2(2566)
General description
Application
- Controlled pore glass beads: Controlled pore glass can be employed for sustainable functionalized metal-organic frameworks for CO2 separation, showcasing the material′s effectiveness in environmental applications due to its high BET surface area which enhances gas adsorption properties (Babar et al., 2021).
- Porous glass for protein adsorption: Mesoporous nano-bioglass for controlled drug release, highlights the application of controlled pore glass with high surface areas in pharmaceutical formulations to enhance the delivery and efficacy of therapeutic agents (Shoaib et al., 2017).
- Surface characterization materials: It can be employed for the contact angle hysteresis of materials with controlled pore structures, to understand the wetting properties of high surface area materials like controlled pore glass, which is critical in various industrial and biomedical applications (Salmas and Androutsopoulos, 2001).
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
49.5 °F - closed cup
flash_point_c
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
An rapid method for the siµLtaneous determination of the Z-drugs or sleep aids: zopiclone, zolpidem, and zaleplon is presented here. The need for greater analytical capacity and throughput for the analysis of sleep aid medicines (Z-drugs) in forensic toxicology laboratories can be met by the use of fast Ascentis Express 2.0 micron Fused Core UHPLC Columns.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service