1.00800
Tryptic Soy Broth - Dehydrated Culture Media
GranuCult® prime, EP, JP, USP, granular, irradiated, suitable for aseptic process simulation
Synonym(s):
Casein-Peptone soymeal-peptone broth, Soybean casein digest broth, TSB, CASO, Casein Soybean Digest Broth, Tryptic Soy Broth, Tryptone Soya Broth, SCDB, CASO Broth, Casein Soya Broth, Soybean Casein digest Broth, TSB, Tryptone Soya Broth
About This Item
Recommended Products
agency
EP
JP
USP
Quality Level
sterility
irradiated
form
granular
feature
granulated
packaging
pkg of 5 kg
pkg of 500 g
manufacturer/tradename
GranuCult® prime
technique(s)
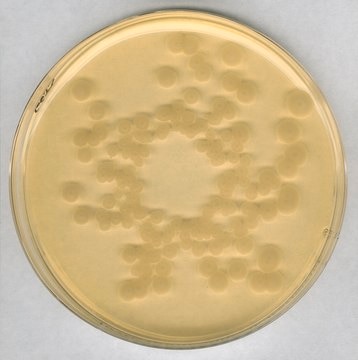
media fill: suitable
microbiological culture: suitable
solubility
30 g/L
bulk density
570 kg/m3
application(s)
aseptic process simulation
pharmaceutical
compatibility
stand alone
storage temp.
15-25°C
suitability
anaerobic bacteria
Looking for similar products? Visit Product Comparison Guide
General description
Application
Linkage
Other Notes
Footnote
The designations basic, plus, or prime are added to indicate the quality control level, from basic quality control to standard QC plus to prime for full regulatory compliance.
Legal Information
wgk_germany
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
High quality granulated and ready-to-use culture media irradiated for accurate and reliable aseptic process simulations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service