679101
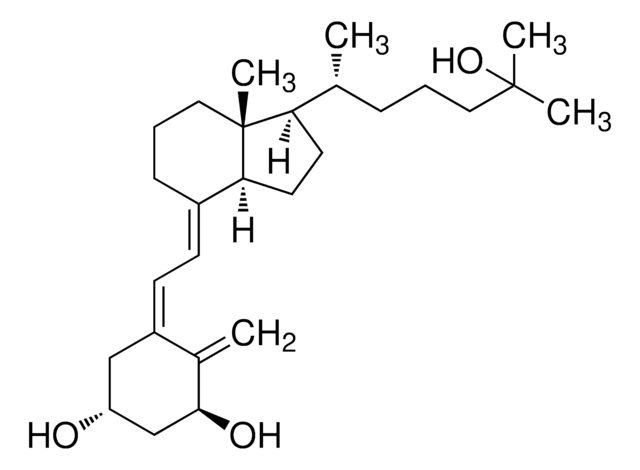
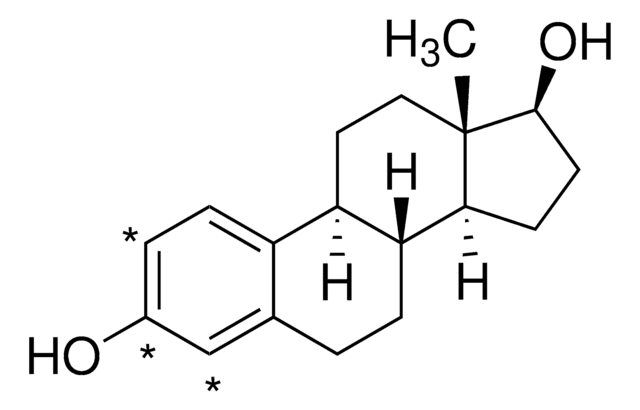
Vitamin D3, 1α, 25-Dihydroxy-
CAS 32222-06-3 prevents the development of clinical diabetes in NOD mice, an animal model of human autoimmune diabetes.
Synonym(s):
Vitamin D3, 1α, 25-Dihydroxy-, 1,25-(OH)₂-D₃, Calcitriol
About This Item
Recommended Products
Quality Level
assay
≥95% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
solubility
DMSO: 50 mg/mL
ethanol: soluble
shipped in
wet ice
storage temp.
−70°C
InChI
1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1
InChI key
GMRQFYUYWCNGIN-NKMMMXOESA-N
Related Categories
General description
Application
- Vitamin D deficiency or resistance and hypophosphatemia.: Discusses the complex interplay between vitamin D metabolism and phosphate homeostasis, emphasizing the crucial role of 1α,25-Dihydroxy-vitamin D3 in managing and understanding bone mineral disorders and their systemic implications (Sarathi et al., 2024).
- Altered Expression of Vitamin D Metabolism Genes and Circulating MicroRNAs in PBMCs of Patients with Type 1 Diabetes.: Investigates how 1α,25-Dihydroxy-vitamin D3 influences gene expression and microRNA profiles in peripheral blood mononuclear cells, linking vitamin D status with autoimmune processes in diabetes (Al-Nakhle et al., 2023).
- 1α,25-Dihydroxyvitamin D(3) Improves Follicular Development and Steroid Hormone Biosynthesis by Regulating Vitamin D Receptor in the Layers Model.: Demonstrates the role of 1α,25-Dihydroxy-vitamin D3 in enhancing reproductive functions and hormonal balance through its action on the vitamin D receptor, providing insights into fertility management (Cheng et al., 2023).
- 1α,25(OH)(2)D(3) Promotes the Autophagy of Porcine Ovarian Granulosa Cells as a Protective Mechanism against ROS through the BNIP3/PINK1 Pathway.: This study presents the protective effects of 1α,25-Dihydroxy-vitamin D3 on ovarian cells under oxidative stress, suggesting mechanisms that could be exploited for therapeutic interventions in reproductive health (Wang et al., 2023).
Packaging
Warning
Reconstitution
Other Notes
Hannun, Y.A. 1994. J. Biol. Chem.269, 3125.
Mathieu, C., et al. 1994. Diabetologica37, 552.
Thomasset, M. 1994. Pathol. Biol. 42, 163.
Horton, W.E., Jr., et al. 1991. J. Biol. Chem.266, 24804.
Larsen, C.G., et al. 1991. Biochem. Biophys. Res. Commun.176, 1020.
Thavarajah, M., et al. 1991. Biochem. Biophys. Res. Commun.176, 1189.
Wakasugi, M., et al. 1991. Prostaglandins42, 127.
Matsumoto, K., et al. 1990. Biochem. Biophys. Res. Commun.166, 916.
Simpson, R.U., et al. 1989. J. Biol. Chem.264, 19710.
DeLuca, H.F., and Schnoes, H.K. 1983. Annu. Rev. Biochem.52, 411.
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 1 Oral - Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - STOT RE 1
Storage Class
6.1A - Combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service