General description
Bromodeoxyuridine (BrdU) is a thymidine analog that, when added exogenously, is incorporated into DNA during DNA synthesis. Anti-bromodeoxyuridine monoclonal antibody is used to label proliferating cells that have incorporated BrdU. This immunological detection scheme has several advantages over the use of radioactive thymidine incorporation for identifying cells undergoing replication. Labeling and detection can be performed the same day instead of waiting several days, as required for autoradiography of tritium-labeled cells, and the necessity of using multiple specimens for obtaining the optimal exposure time is eliminated. In addition, anti-bromodeoxyuridine staining with flow cytometric analysis allows multiple parameters to be evaluated simultaneously.
Specificity
Clone BU-1 specifically binds to bromodeoxyuridine with 10% crossreactivity toward iodouridine (10%). Clone BU-1 does not crossreact with fluorodeoxyuridine or any endogenous cellular components such as thymidine or uridine.
Immunogen
Bromodeoxyuridine
Epitope: BrdU
Application
Although originally reported to stain nuclear DNA BrdU incorporation in ethanol-fixed cells without acid or base denaturation, this property was later attributed to the DNase activity present in a mycoplasma-contaminated BU-1 hybridoma culture supernatant used (Gonchoroff, N.J., et al. (1986). J. Immunol. Methods. 93(1):97-101; Greipp, P.R., et al. (1985). Am. J. Hematol. 20(3):289-292). Please include an appropriate DNA denaturation step during sample preparation prior to immunostaining using this product.
Research Category
Epigenetics & Nuclear Function
Research Sub Category
Cell Cycle, DNA Replication & Repair
Use mouse monoclonal Anti-BrdU Antibody, clone BU-1, validated in Flow Cytometry, Immunocytochemistry, and Immunohistochemistry for the detection of bromodeoxyuridine (BrdU).
Physical form
Hybridoma culture supernatant from a perfusion system, filtered through a 0.2 micron membrane prior to vialing. Product contains 20% FBS and Ciprofloxacin at a final concentration of 10 μg/mL. Possible mycoplasma risk contained within this product. Use appropriate measures to prevent cell line infection/contamination.
Unpurified
Storage and Stability
Maintain for 1 year at -20°C from date of shipment. Aliquot to avoid repeated freezing and thawing. For maximum recovery of product, centrifuge the original vial after thawing and prior to removing the cap.
Analysis Note
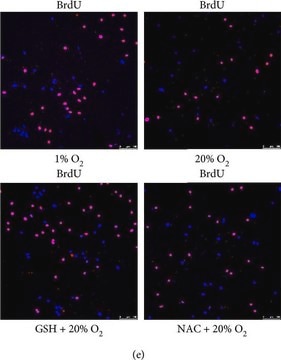
Control
Brdu treated cells
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.