LMDH-RO
Roche
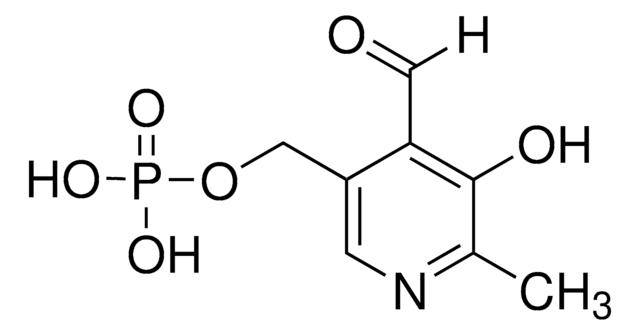
L-Malate Dehydrogenase (L-MDH)
from pig heart (mitochondrial)
About This Item
Recommended Products
biological source
Porcine heart (mitochondrial)
Quality Level
form
solution
suspension
specific activity
~1200 units/mg protein (At 25 °C with oxaloacetate as the substrate.)
packaging
pkg of 1 mL (10127248001 solution)
pkg of 1 mL (10127256001 suspension)
pkg of 5 mL (10127914001suspension)
manufacturer/tradename
Roche
technique(s)
activity assay: suitable
color
white
optimum pH
7.4-7.5(reduction of oxaloacetate)
9.2-9.5(oxidation of malate)
solubility
water: miscible
suitability
suitable for Western blot
NCBI accession no.
UniProt accession no.
application(s)
life science and biopharma
foreign activity
Fumarase <0.01%
GIDH 0.001%
GOT 0.001%
GPT 0.00185%
LDH <0.01%
NADH oxidase <0.001%
shipped in
wet ice
storage temp.
2-8°C
Gene Information
Porcine ... MDH2(397039)
Related Categories
General description
The L-malate dehydrogenase enzyme is a nuclear gene product that is synthesized with a 24-residue amino-terminal signal peptide. This peptide is proteolytically cleaved during the translocation of the enzyme to the mitochondrial matrix.
Application
Biochem/physiol Actions
Quality
Physical form
Preparation Note
– arsenate
– Mg2+
– Zn2+
Other Notes
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
No data available
flash_point_c
No data available
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service