439142
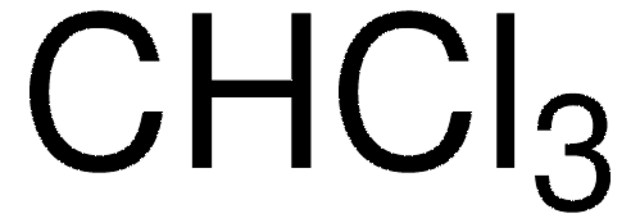
Chloroform
suitable for HPLC, ≥99.8%, contains 0.5-1.0% ethanol as stabilizer
Synonym(s):
Methylidyne trichloride, Trichloromethane
About This Item
Recommended Products
vapor density
4.1 (vs air)
Quality Level
vapor pressure
160 mmHg ( 20 °C)
assay
≥99.8%
form
liquid
contains
0.5-1.0% ethanol as stabilizer
packaging
PVC-coated bottle of 4, 4x4 L
technique(s)
HPLC: suitable
impurities
<0.01% water
evapn. residue
<0.0003%
color
APHA: ≤10
refractive index
n20/D 1.445 (lit.)
bp
60.5-61.5 °C (lit.)
mp
−63 °C (lit.)
density
1.48 g/mL at 25 °C
1.492 g/mL at 25 °C (lit.)
cation traces
Pb: ≤0.05 ppm
λ
H2O reference
UV absorption
λ: 245 nm Amax: 1.00
λ: 255 nm Amax: 0.15
λ: 260 nm Amax: 0.15
λ: 270 nm Amax: 0.02
λ: 290-400 nm Amax: 0.01
application(s)
food and beverages
SMILES string
ClC(Cl)Cl
InChI
1S/CHCl3/c2-1(3)4/h1H
InChI key
HEDRZPFGACZZDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
It may be used as:
- Solvent for electropolymerization.
- Solvent for recrystallization.
- Extractant in solvent extraction process.
signalword
Danger
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 Oral - STOT SE 3
target_organs
Central nervous system
Storage Class
6.1D - Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
wgk_germany
WGK 3
flash_point_f
does not flash
flash_point_c
does not flash
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service