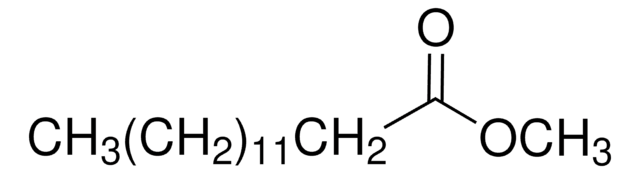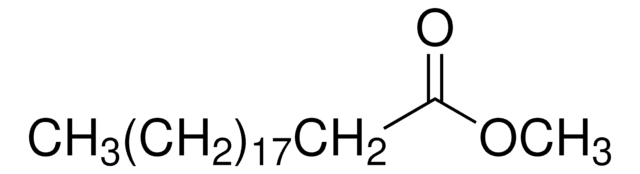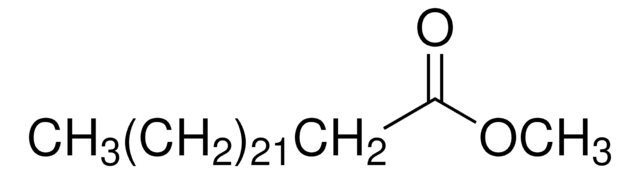70129
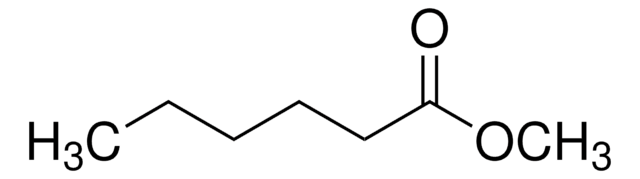
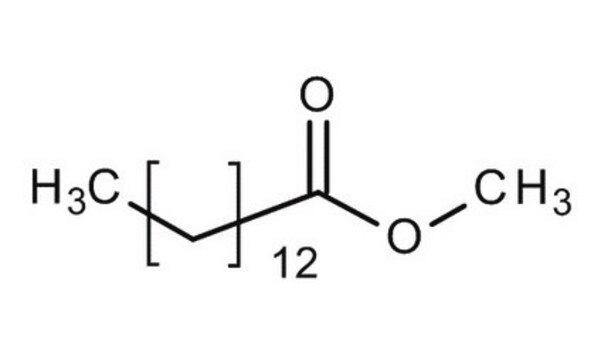
Methyl myristate
analytical standard
Synonym(s):
Methyl tetradecanoate, Myristic acid methyl ester
About This Item
Recommended Products
grade
analytical standard
Quality Level
assay
≥99.5% (GC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.436 (lit.)
n20/D 1.438
bp
323 °C (lit.)
mp
18 °C (lit.)
density
0.855 g/mL at 25 °C (lit.)
format
neat
functional group
ester
shipped in
ambient
storage temp.
room temp
SMILES string
CCCCCCCCCCCCCC(=O)OC
InChI
1S/C15H30O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15(16)17-2/h3-14H2,1-2H3
InChI key
ZAZKJZBWRNNLDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Comparative analysis of gas chromatography-combustion-mass spectrometry and gas chromatography-flame ionization detector methods for the determination of fatty acid methyl esters (FAMEs) in biodiesel samples
- Gas chromatography-tandem differential mobility spectrometry (DMS) based separation and quantification of 16 methyl- and ethyl- fatty acid esters from biodiesel samples
- Analysis of coffee oil and residue obtained from roasted coffee beans to determine the composition of 11 fatty acids following their methyl esterification by gas chromatography coupled with a flame ionization detector (GC-FID)
- Simultaneous determination of fatty acids in bovine colostrum samples by GC-FID after their derivatization to ester forms using an acidic catalyst boron trifluoride
- Separation and quantification of eight fatty acids after their derivatization to methyl esters in the oil extracted from the leaves of Abutilon hirtum (Lam.) by GC-MS
Other Notes
Recommended products
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
233.6 °F - closed cup
flash_point_c
112.0 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Separation of Methyl erucate; Methyl palmitate; Methyl stearate; Methyl linolenate; Methyl eicosenoate; Methyl behenate; Methyl myristate; Methyl oleate; Methyl arachidate
Protocol for GC Analysis of Bacterial Acid Methyl Esters (BAMEs) on Equity®-1
Separation of Methyl decanoate; Methyl dodecanoate; Methyl myristate; Methyl palmitate; Methyl caprylate; Methyl oleate; Methyl linoleate; Methyl linolenate; Methyl stearate
-11-eicosenoate; Methyl elaidate; Methyl linoleate; Methyl myristate; Methyl myristoleate; Methyl palmitate; Methyl palmitoleate; Methyl oleate; Methyl pentadecanoate; Methyl tridecanoate; Methyl behenate; Methyl caprylate; Methyl erucate; Methyl heptadecanoate; Methyl arachidate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service