A9085
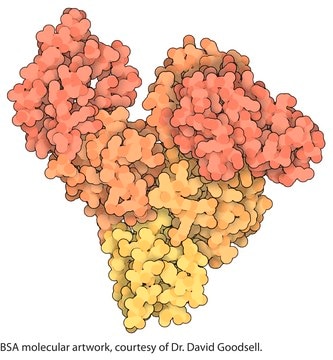
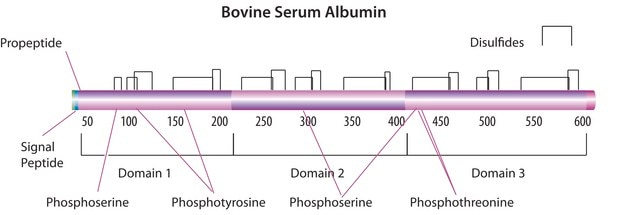
Bovine Serum Albumin
lyophilized powder, essentially IgG free, ≥96% (agarose gel electrophoresis)
Synonym(s):
Albumin bovine serum, BSA, Bovine albumin
About This Item
Recommended Products
biological source
bovine
Quality Level
assay
≥96% (agarose gel electrophoresis)
form
lyophilized powder
mol wt
~66 kDa
purified by
heat shock fractionation
packaging
poly bottle of
origin
USA origin
technique(s)
flow cytometry: suitable
immunofluorescence: suitable
impurities
IgG free
≤25 ng/mg protein IgG
pH
7
solubility
water: soluble (40 mg/ml)
UniProt accession no.
foreign activity
Blue tongue virus, none detected
Vesicular Statitis virus, none detected
storage temp.
2-8°C
Gene Information
bovine ... ALB(280717)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- the washing of the rabbit tissue sections in immunofluorescence microscopy
- incubation of red blood cells (RBC) for flow cytometry analysis
- as a component of Hanks′ balanced salt solution with Ca2+ and Mg2+ (HBSS++) for eosin-5-maleimide staining and RBC washing in magnetic circular dichroism measurements
Biochem/physiol Actions
Features and Benefits
- Essentially IgG free (immunoglobulin-free)
- Heat shock fractionated
Preparation Note
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service