PHR1000
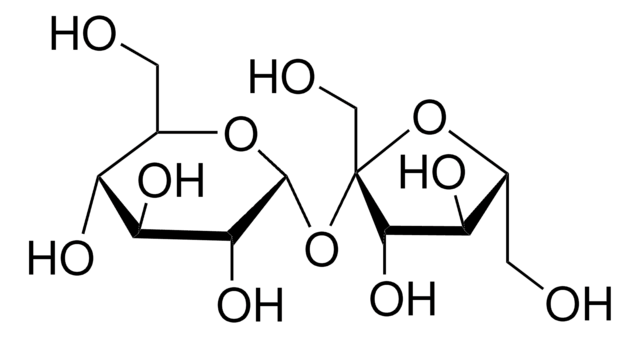
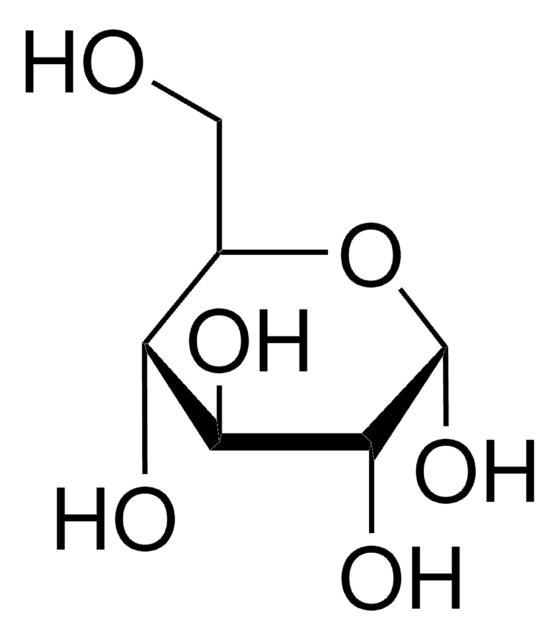
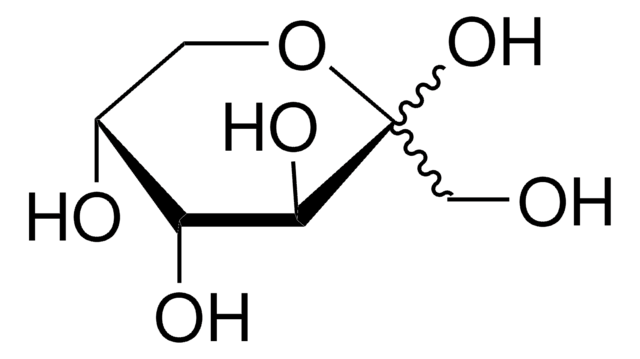
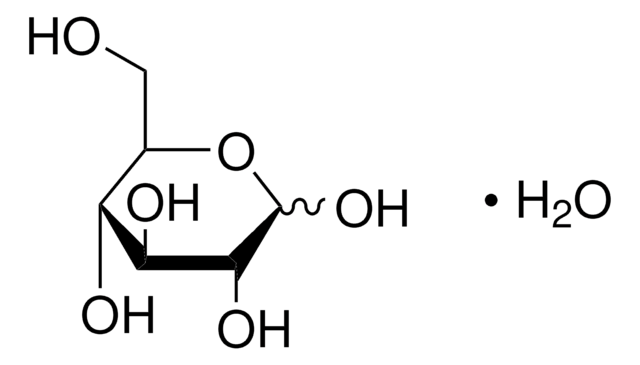
Dextrose
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
D-(+)-Glucose, Dextrose
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to Ph. Eur. Y0001745
traceable to USP 1181302
API family
dextrose
assay
99.9%
CofA
current certificate can be downloaded
analyte chemical class(es)
oligosaccharides
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
150-152 °C (lit.)
application(s)
detection
food and beverages
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6+/m1/s1
InChI key
WQZGKKKJIJFFOK-DVKNGEFBSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Application
Analysis Note
Other Notes
Footnote
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Separation of Dextrose, Pharmaceutical Secondary Standard; Certified Reference Material; Sucrose; Maltitol, Pharmaceutical Secondary Standard; Certified Reference Material
Determination of total glucose and xylose in coffee samples by Reversed Phase High-performance liquid chromatography (RP-HPLC) coupled to UV detector.
Protocols
A protocol discussing HPLC Analysis of Sugars on SUPELCOSIL™ LC-NH2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service