PHR1148
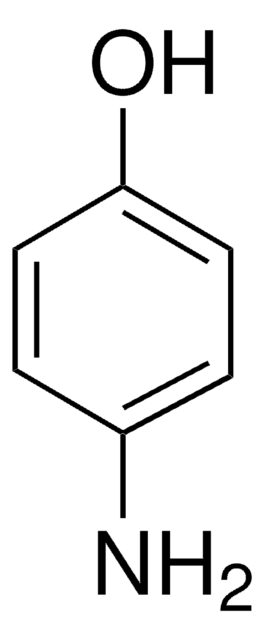
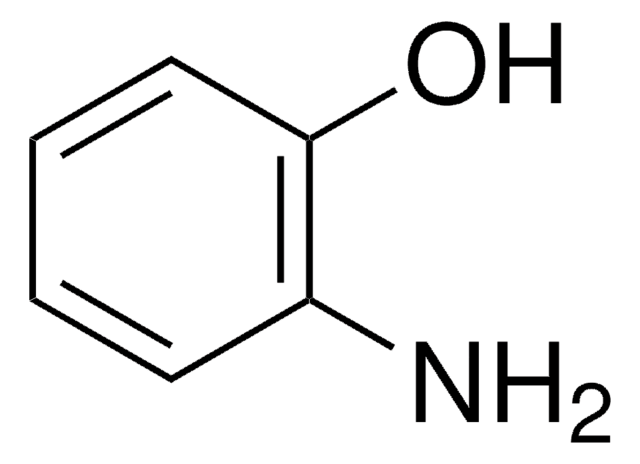
4-Aminophenol (Acetaminophen Related Compound K) (Paracetamol Impurity K)
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
4-Aminophenol, 4-Hydroxyaniline
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to Ph. Eur. Y0001955
traceable to USP 1021204
API family
mesalazine, paracetamol, acetaminophen
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
185-189 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
Nc1ccc(O)cc1
InChI
1S/C6H7NO/c7-5-1-3-6(8)4-2-5/h1-4,8H,7H2
InChI key
PLIKAWJENQZMHA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is a primary degradation product of the widely used antipyretic and analgesic drug acetaminophen, also known as paracetamol, formed on its hydrolysis.
Application
- Separation and estimation of acetaminophen and its process impurities in commercial acetaminophen tablets using high-performance liquid chromatography (HPLC)
- Simultaneous electrochemical determination of acetaminophen and its impurity K using a reduced graphene oxide-titanium nitride (RGO-TiN) nanohybrid
- Development and validation of HPLC-based stability indicating method for the determination of acetaminophen, chlorpheniramine maleate, and their possible degradation products in an over-the-counter syrup formulation
- Determination of acetaminophen, pamabrom, and their impurities using high-performance thin layer chromatography (HPTLC) and reversed phase-high performance liquid chromatography (RP-HPLC), according to the ICH guidelines, in their combined dosage tablets
- Combined analysis of acetaminophen, chlorzoxazone, and their major degradation impurities using thin-layer chromatographic (TLC)-densitometric method in their combined dosage capsules
Analysis Note
Footnote
Recommended products
related product
signalword
Warning
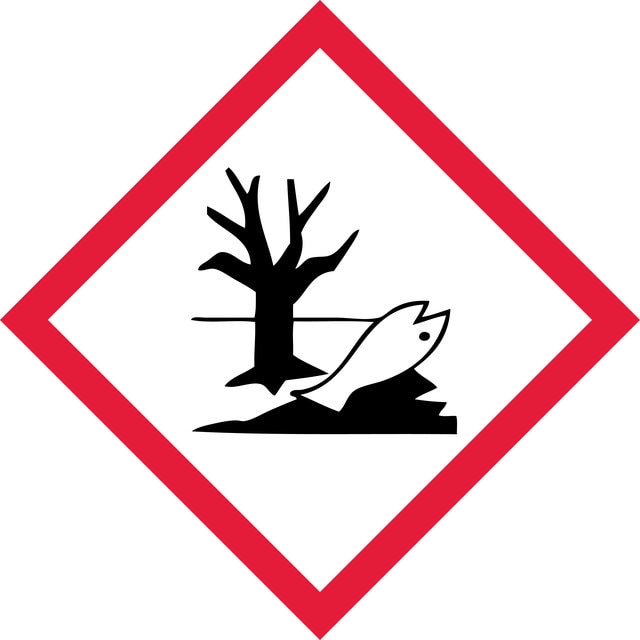
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Muta. 2 - Skin Sens. 1 - STOT RE 2
target_organs
Kidney
Storage Class
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service