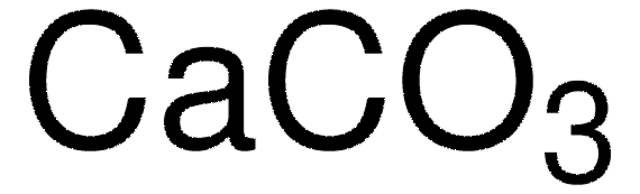All Photos(2)
About This Item
Linear Formula:
CaCO3
CAS Number:
Molecular Weight:
100.09
Beilstein/REAXYS Number:
8008338
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.55
Quality Level
product line
ReagentPlus®
assay
≥99% (EDTA titration)
form
powder
pH
8
density
2.93 g/mL at 25 °C (lit.)
SMILES string
[Ca++].[O-]C([O-])=O
InChI
1S/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI key
VTYYLEPIZMXCLO-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Calcium carbonate is a calcium salt commonly found in shells and skeleton of marine animals. It is an inexpensive, non-toxic and easily available reagent with a wide range of applications such as uptake of CO2 and extraction of heavy metals and arsenic from ground water. Its crystals exist as 3 different polymorphs namely, calcite, aragonite, and vaterite. The kinetics of pyrolysis of calcium carbonate under non-isothermal conditions have been investigated.
Application
Calcium carbonate may be used:
- As a pore generating agent to prepare porous ceramics.
- As chelometric standard for the standardization of EDTA solution.
- To extract aluminium by microwave induced thermal activation of coal fly ash (CFA).
Iron-Catalyzed Efficient Synthesis of Amides from Aldehydes and Amine Hydrochloride Salts
Iron-Catalyzed Efficient Synthesis of Amides from Aldehydes and Amine Hydrochloride Salts
Iron-Catalyzed Efficient Synthesis of Amides from Aldehydes and Amine Hydrochloride Salts
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
13 - Non Combustible Solids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kinetics of calcium carbonate decomposition.
Rao TR.
Chemical Engineering & Technology, 19(4), 373-377 (1996)
Calcium carbonate nanoparticles as cancer drug delivery system.
Maleki Dizaj S, et al.
Expert Opinion on Drug Delivery, 12(10) (2015)
Opposite particle size effect on amorphous calcium carbonate crystallization in water and during heating in air.
Zou Z, et al.
Chemistry of Materials, 27(12), 4237-4246 (2015)
Aluminum release from microwave-assisted reaction of coal fly ash with calcium carbonate.
Zhang ZY, et al.
Fuel Processing Technology, 134, 303-309 (2015)
Permeability of porous ceramic based on calcium carbonate as pore generating agent.
Sim?o L, et al.
Ceramics International, 41(3), 4782-4788 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service