42418
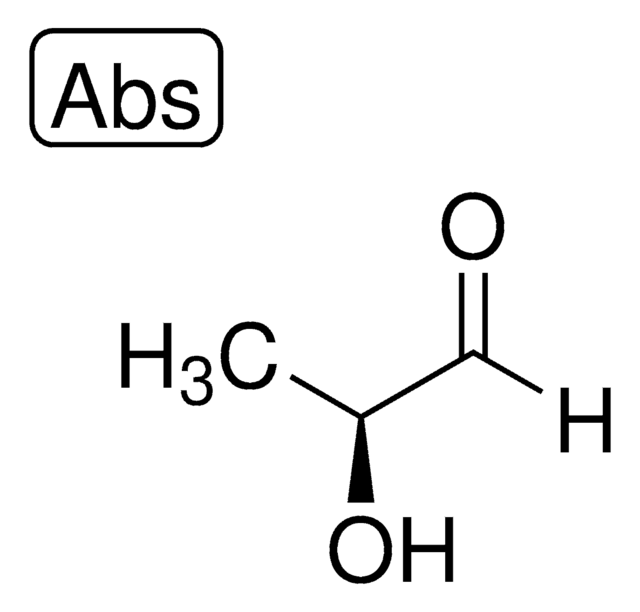
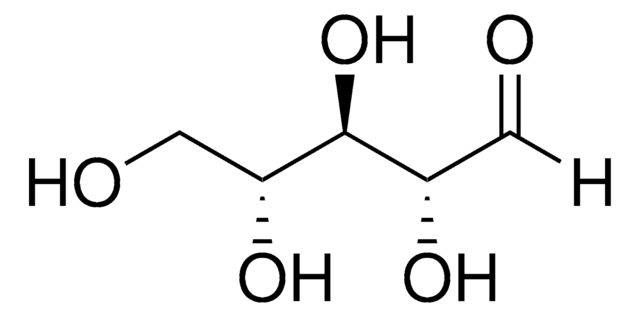
D-Lactaldehyde solution
1 M in H2O
Synonym(s):
(2R)-2-Hydroxypropanal solution, (R)-2-Hydroxypropionaldehyde solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
assay
≥95.0% (TLC)
Quality Level
form
liquid
optical purity
enantiomeric ratio: ≥98.0:2.0 (HPLC)
concentration
1 M in H2O
storage temp.
−20°C
SMILES string
C[C@@H](O)C([H])=O
InChI
1S/C3H6O2/c1-3(5)2-4/h2-3,5H,1H3/t3-/m1/s1
InChI key
BSABBBMNWQWLLU-GSVOUGTGSA-N
Biochem/physiol Actions
D-Lactaldehyde is an intermediate in the pyruvate metabolic pathway. Pyruvaldehyde is irreversibly produced from D-lactaldehyde via the enzyme glyoxylate reductase.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P J Thornalley
Amino acids, 6(1), 15-23 (1994-02-01)
The formation of the reactiveα,β-dicarbonyl metabolite, methylglyoxal, is increased during hyperglycaemia associated with diabetes mellitus. Methylglyoxal is metabolised to S-D-lactoylglutathione and D-lactate by the glyoxalase system and to hydroxyacetone (95%) and D-lactaldehyde by aldose reductase. Methylglyoxal and hydroxyacetone bind and
D-Fucose metabolism in a pseudomonad. IV. Cleavage of 2-keto-3-deoxy-D-fuconate to pyruvate and D-lactaldehyde by 2-keto-3-deoxy-L-arabonate aldolase.
A S Dahms et al.
The Journal of biological chemistry, 247(7), 2238-2241 (1972-04-10)
THE METABOLISM OF LACTALDEHYDE. VII. THE OXIDATION OF D-LACTALDEHYDE IN RAT LIVER.
S M TING et al.
Biochimica et biophysica acta, 97, 407-415 (1965-03-08)
D L Vander Jagt et al.
The Journal of biological chemistry, 267(7), 4364-4369 (1992-03-05)
The substrate specificities of human aldose reductase and aldehyde reductase toward trioses, triose phosphates, and related three-carbon aldehydes and ketones were evaluated. Both enzymes are able to catalyze the NADPH-dependent reduction of all of the substrates used. Aldose reductase shows
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service