G7509
GABase from Pseudomonas fluorescens
lyophilized powder, Protein ≥30 % by biuret
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Pseudomonas fluorescens
Quality Level
assay
≥30 % (biuret)
form
lyophilized powder
specific activity
≥0.5 units/mg protein
composition
Protein, ≥30% biuret
technique(s)
inhibition assay: suitable
storage temp.
−20°C
General description
Research area: Neuroscience
GABase is a commercially available mixture composed of γ-aminobutyric acid aminotransferase (GABGT) and succinic semialdehyde dehydrogenase (SSDH) obtained from Pseudomonas fluorescens.
GABase is a commercially available mixture composed of γ-aminobutyric acid aminotransferase (GABGT) and succinic semialdehyde dehydrogenase (SSDH) obtained from Pseudomonas fluorescens.
Application
GABase from Pseudomonas fluorescens has been used for GABase assay to quantify the extracellular and intracellular concentration of GABA (γ-aminobutyric acid).
Biochem/physiol Actions
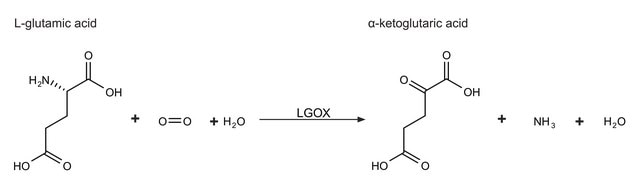
GABase catalyzes the conversion of γ-aminobutyric acid (GABA) to succinic acid in the central nervous system. It is used to measure GABA levels in various biological samples. p-Hydroxybenzaldehyde (HBA) is known to be a potential inhibitor on GABase.
Unit Definition
One unit will convert 1.0 μmole of γ-aminobutyric acid (GABA) to succinic semialdehyde and then to succinate per min with a stoichiometric reduction of 1.0 μmole of NADP+ at pH 8.6 at 25 °C.
Physical form
Partially purified lyophilized powder containing buffer salts and stabilizer
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kimon-Andreas G Karatzas et al.
Applied and environmental microbiology, 76(11), 3529-3537 (2010-04-20)
It is well established that the glutamate decarboxylase (GAD) system is central to the survival of Listeria monocytogenes at low pH, both in acidic foods and within the mammalian stomach. The accepted model proposes that under acidic conditions extracellular glutamate
A Vidal-Cros et al.
The Biochemical journal, 229(3), 675-678 (1985-08-01)
L-threo-3-Fluoroglutamate and L-erythro-3-fluoroglutamate were tested with glutamate decarboxylase from Escherichia coli. Both isomers were substrates: the threo isomer was decarboxylated into optically active 4-amino-3-fluorobutyrate, whereas the erythro isomer lost the fluorine atom during the reaction, yielding succinic semialdehyde after hydrolysis
Mingu Gordon Park et al.
Journal of enzyme inhibition and medicinal chemistry, 36(1), 2016-2024 (2021-09-14)
Many studies have focussed on modulating the activity of γ-aminobutyric acid transaminase (GABA-T), a GABA-catabolizing enzyme, for treating neurological diseases, such as epilepsy and drug addiction. Nevertheless, human GABA-T synthesis and purification have not been established. Thus, biochemical and drug
Hexigeduleng Bao et al.
Plant, cell & environment, 38(3), 600-613 (2014-07-31)
γ-Aminobutyric acid (GABA) accumulates in many plant species in response to environmental stress. However, the physiological function of GABA or its metabolic pathway (GABA shunt) in plants remains largely unclear. Here, the genes, including glutamate decarboxylases (SlGADs), GABA transaminases (SlGABA-Ts) and
Alejandra Hurtado-Romero et al.
Foods (Basel, Switzerland), 10(10) (2021-10-24)
Isolation and functional characterization of microorganisms are relevant steps for generating starter cultures with functional properties, and more recently, those related to improving mental health. Milk kefir grains have been recently investigated as a source of health-related strains. This study
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service