GS63
GST A3-3, Recombinant Human
Synonym(s):
GTA3, glutathione S-transferase alpha 3
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.26
Recommended Products
biological source
human
recombinant
expressed in E. coli
assay
>95% (SDS-PAGE)
form
frozen liquid
specific activity
61 U/mg
mol wt
25 kDa
concentration
1.0 mg/mL
storage temp.
−70°C
Gene Information
human ... GSTA3(2940)
General description
using spectrophotometric determination of 1-chloro-2,4-dinitrobenzene (CDNB) conjugation with reduced glutathione (1 mM) in 100 mM NaPO4 (pH 6.5) at room temperature.
Biochem/physiol Actions
Glutathione S-transferase alpha 3 (GSTA3) is an enzyme that in humans is encoded by the GSTA3 gene. Glutathione S-transferases (GSTs) are a family of enzymes that play an important role in detoxification by catalyzing the conjugation of many hydrophobic and electrophilic compounds with reduced glutathione. Based on their biochemical, immunologic, and structural properties, cytosolic and membrane-bound forms of glutathione S-transferase are encoded by two distinct supergene families. At present, eight distinct classes of the soluble cytoplasmic mammalian glutathione S-transferases have been identified: alpha, kappa, mu, omega, pi, sigma, theta and zeta. The GSTs are thought to function in xenobiotic metabolism and play a role in susceptibility to cancer, and other diseases.
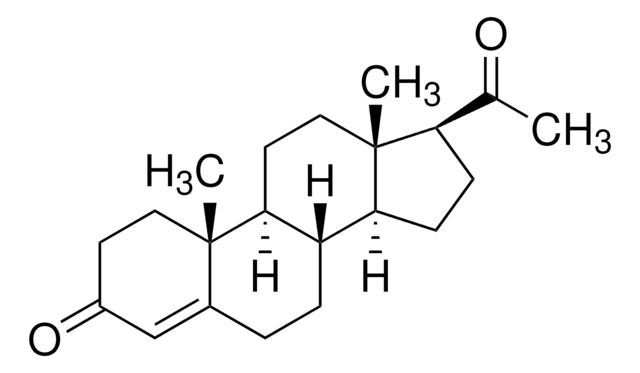
This gene encodes a glutathione S-tranferase belonging to the alpha class genes that are located in a cluster mapped to chromosome 6. Genes of the alpha class are highly related and encode enzymes with glutathione peroxidase activity. However, during evolution, this alpha class gene diverged accumulating mutations in the active site that resulted in differences in substrate specificity and catalytic activity. The enzyme encoded by this gene catalyzes the double bond isomerization of precursors for progesterone and testosterone during the biosynthesis of steroid hormones. An additional transcript variant has been identified, but its full length sequence has not been determined.
Storage and Stability
The enzyme should be used by the end-user customer within 1 year of receipt.
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jonathan L Daka et al.
The Journal of biological chemistry, 289(46), 32243-32252 (2014-09-25)
The seemingly simple proton abstraction reactions underpin many chemical transformations, including isomerization reactions, and are thus of immense biological significance. Despite the energetic cost, enzyme-catalyzed proton abstraction reactions show remarkable rate enhancements. The pathways leading to these accelerated rates are
Ann-Sofie Johansson et al.
The Journal of biological chemistry, 277(19), 16648-16654 (2002-03-02)
Glutathione transferase (GST) A3-3 is the most efficient human steroid double-bond isomerase known. The activity with Delta(5)-androstene-3,17-dione is highly dependent on the phenolic hydroxyl group of Tyr-9 and the thiolate of glutathione. Removal of these groups caused an 1.1 x
Françoise Raffalli-Mathieu et al.
The Biochemical journal, 414(1), 103-109 (2008-04-23)
hGSTA3-3 (human Alpha-class glutathione transferase 3-3) efficiently catalyses steroid Delta(5)-Delta(4) double-bond isomerization in vitro, using glutathione as a cofactor. This chemical transformation is an obligatory reaction in the biosynthesis of steroid hormones and follows the oxidation of 3beta-hydroxysteroids catalysed by
Yijun Gu et al.
Biochemistry, 43(50), 15673-15679 (2004-12-15)
The crystal structure of human class alpha glutathione (GSH) S-transferase A3-3 (hGSTA3-3) in complex with GSH was determined at 2.4 A. Despite considerable amino acid sequence identity with other human class alpha GSTs (e.g., hGSTA1-1), hGSTA3-3 is unique due to
Natasha Tetlow et al.
Pharmacogenetics, 14(10), 657-663 (2004-09-30)
The alpha class glutathione transferase GSTA3-3 is involved in steroid biosynthesis and the metabolism of some xenobiotics. A bioinformatics approach was utilized to identify novel coding region polymorphisms in the glutathione transferase A3 gene (GSTA3). We describe an I71L polymorphism
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service