All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H6N2O2
CAS Number:
Molecular Weight:
126.11
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
assay
≥98% (TLC)
Quality Level
form
powder
solubility
1 M NaOH: 50 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
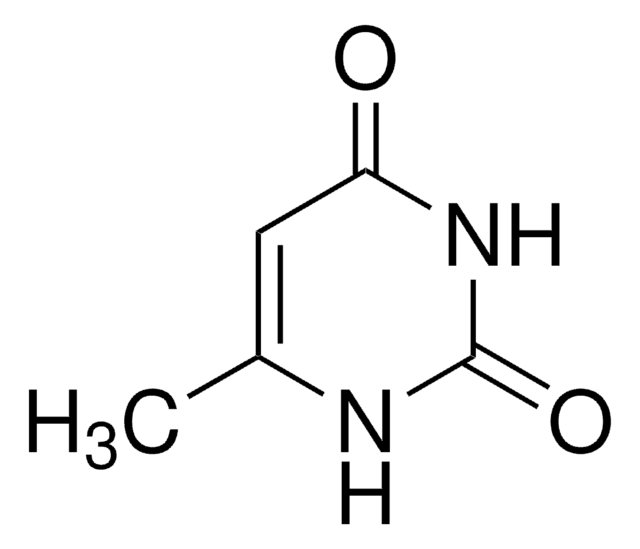
SMILES string
CN1C(=O)NC=CC1=O
InChI
1S/C5H6N2O2/c1-7-4(8)2-3-6-5(7)9/h2-3H,1H3,(H,6,9)
InChI key
VPLZGVOSFFCKFC-UHFFFAOYSA-N
General description
3-Methyluracil is a methylated uracil or a uracil derivative.
Application
3-Methyluracil (3-MeU) is used along with other methyl-pyrimidines to study relative physical chemical parameters.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T V Chestnova et al.
Bulletin of experimental biology and medicine, 165(6), 777-780 (2018-10-26)
Bacterial biofilms provoke and/or promote the most chronic and recurrent infectious diseases. Previously, experimental models of purulent peritonitis and meningoencephalitis revealed positive antibiofilm effect of metallic nanoparticles and the absence of resistance against such nanoparticles in microorganisms. This study examines
Vibrational Feshbach resonances in uracil and thymine.
Burrow PD, Gallup GA, et al.
J. Chem. Phys. , 28, 124310-124310 (2006)
Mihajlo Etinski et al.
Physical chemistry chemical physics : PCCP, 12(19), 4915-4923 (2010-05-07)
In this work we investigated the lowest-lying electronic excitations for a series of methyl-substituted uracil derivatives, i.e., uracil, 1-methyluracil, 3-methyluracil, thymine, 1-methylthymine, 1,3-dimethyluracil, 3-methylthymine, 1,3-dimethylthymine, and their microhydrated complexes by means of coupled cluster singles and approximate doubles (CC2) and
Guifang Jia et al.
FEBS letters, 582(23-24), 3313-3319 (2008-09-09)
The human obesity susceptibility gene, FTO, encodes a protein that is homologous to the DNA repair AlkB protein. The AlkB family proteins utilize iron(II), alpha-ketoglutarate (alpha-KG) and dioxygen to perform oxidative repair of alkylated nucleobases in DNA and RNA. We
Anna Zhachkina et al.
Journal of the American Chemical Society, 131(51), 18376-18385 (2009-11-26)
The gas-phase substitution reactions of methyl chloride and 1,3-dimethyluracil (at the N1-CH(3)) are examined computationally and experimentally. It is found that, although hydrochloric acid and 3-methyluracil are similar in acidity, the leaving group abilities of chloride and N1-deprotonated 3-methyluracil are
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service