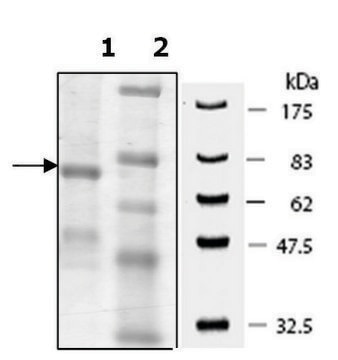
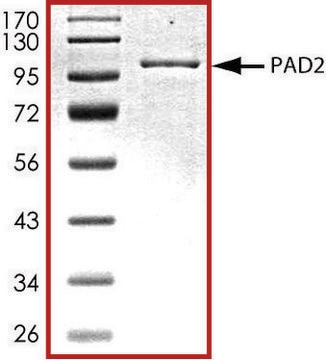
SAE0061
Peptidyl arginine deiminase type-2 (PAD2) human
recombinant, expressed in E. coli
Synonym(s):
PAD-2, PAD2, PADI-2, PADI2
Sign Into View Organizational & Contract Pricing
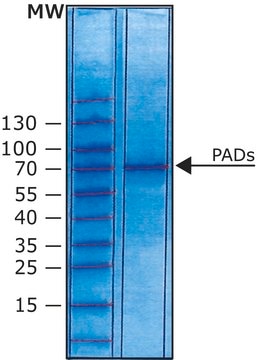
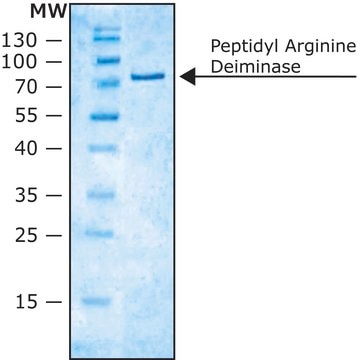
All Photos(2)
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
specific activity
≥40 units/mg protein
shipped in
dry ice
storage temp.
−20°C
General description
Peptidyl Arginine Deiminases (PADs) perform post-translational deiminations of proteins. PADs are calcium-dependent enzymes that catalyze the conversion of L-arginine residues to L-citrulline. The reaction catalyzed is as follows:
Protein--[L-arginine] + H2O+Ca+2→Protein--[L-citrulline]+ NH4
This deimination provides another level of regulating protein function.
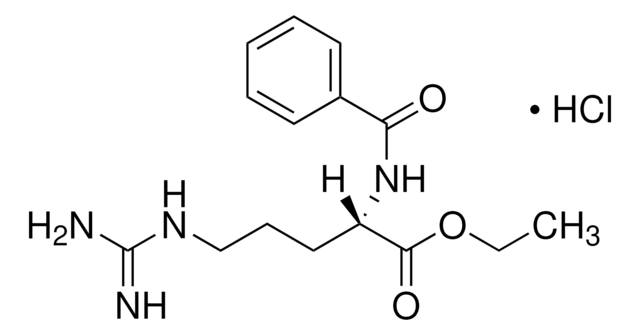
There are five mammalian PADs sub-types, which differ in substrate specificity and tissue distribution. PAD enzymes are highly homologous, with 50–60% sequence similarity. PADenzymes play important roles in gene regulation by citrullination of arginine residues on histones H3, H2A, and H4. Overexpression of these enzymes has been found in several diseases such as rheumatoid arthritis, Alzheimer′s disease, multiple sclerosis, lupus, Parkinson′s disease, and cancer. PAD2 is widely expressed in the brain, secretory glands, and skeletal muscles. The enzyme was found to be responsible for hyper-citrullination of myelin basic protein, which is suspected to lead to multiple sclerosis. The product is supplied in 20 mM Tris, pH 7.5, 200 mM NaCl, 10% (w/v) glycerol, 10 mM 2-mercaptoethanol, 1 mM EDTA, 1 mM DTT, 10 mM CaCl2, 10 mM arginine, 30 mM maltose, and 0.6 mg/ml dodecyl−β-D-maltoside. Enzymatic activity: Activity is measured by a colorimetric method with the synthetic substrate benzoyl arginine ethyl ester (BAEE).
Protein--[L-arginine] + H2O+Ca+2→Protein--[L-citrulline]+ NH4
This deimination provides another level of regulating protein function.
There are five mammalian PADs sub-types, which differ in substrate specificity and tissue distribution. PAD enzymes are highly homologous, with 50–60% sequence similarity. PADenzymes play important roles in gene regulation by citrullination of arginine residues on histones H3, H2A, and H4. Overexpression of these enzymes has been found in several diseases such as rheumatoid arthritis, Alzheimer′s disease, multiple sclerosis, lupus, Parkinson′s disease, and cancer. PAD2 is widely expressed in the brain, secretory glands, and skeletal muscles. The enzyme was found to be responsible for hyper-citrullination of myelin basic protein, which is suspected to lead to multiple sclerosis. The product is supplied in 20 mM Tris, pH 7.5, 200 mM NaCl, 10% (w/v) glycerol, 10 mM 2-mercaptoethanol, 1 mM EDTA, 1 mM DTT, 10 mM CaCl2, 10 mM arginine, 30 mM maltose, and 0.6 mg/ml dodecyl−β-D-maltoside. Enzymatic activity: Activity is measured by a colorimetric method with the synthetic substrate benzoyl arginine ethyl ester (BAEE).
Unit Definition
One unit will produce 1.0 μmole of N-a-benzoylcitrulline ethyl ester from BAEE per hr at 55 °C at pH 7.2.
Physical form
Supplied as a buffered aqueous solution containing Trizma, NaCl, glycerol, mercaptoethanol, EDTA, DTT, CaCl2, arginine, maltose, and dodecyl-β-D-maltoside.
Preparation Note
Contains a maltose-binding protein fusion tag.
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sunish Mohanan et al.
Biochemistry research international, 2012, 895343-895343 (2012-09-29)
The peptidylarginine deiminases (PADs) are a family of posttranslational modification enzymes that catalyze the conversion of positively charged protein-bound arginine and methylarginine residues to the uncharged, nonstandard amino acid citrulline. This enzymatic activity is referred to as citrullination or, alternatively
H Takahara et al.
Journal of biochemistry, 94(6), 1945-1953 (1983-12-01)
The preceding paper described the identification and some properties of peptidylarginine deiminase, which catalyzes the deimination of arginyl residues in protein, from rabbit skeletal muscle, kidney, brain, and lung. In the present work we purified peptidylarginine deiminase from rabbit skeletal
Olena Mahneva et al.
PloS one, 15(1), e0227822-e0227822 (2020-01-16)
Peptidylarginine deiminase (PAD) modifies peptidylarginine and converts it to peptidylcitrulline in the presence of elevated calcium. Protein modification can lead to severe changes in protein structure and function, and aberrant PAD activity is linked to human pathologies. While PAD homologs
Mario A Moscarello et al.
Neurochemical research, 32(2), 251-256 (2006-10-13)
The pathogenesis of MS is unknown. In our studies, we have demonstrated an important role for citrullinated myelin basic protein (MBP). The accompanying loss of positive charge compromises the ability of MBP to interact with the lipid bilayer. The conversion
Kevin L Bicker et al.
Biopolymers, 99(2), 155-163 (2012-11-24)
The post-translational modification of histones has significant effects on overall chromatin function. One such modification is citrullination, which is catalyzed by the protein arginine deiminases (PADs), a unique family of enzymes that catalyzes the hydrolysis of peptidyl-arginine to form peptidyl-citrulline
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service