SML0341
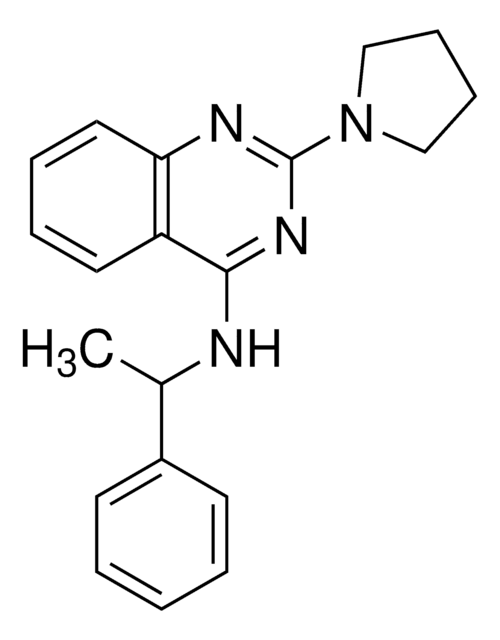
Importazole
≥98% (HPLC)
Synonym(s):
N-(1-Phenylethyl)-2-(pyrrolidin-1-yl)quinazolin-4-amine
About This Item
Recommended Products
Quality Level
assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: >5 mg/mL
storage temp.
2-8°C
SMILES string
CC(C1=CC=CC=C1)NC2=NC(N3CCCC3)=NC4=C2C=CC=C4
InChI
1S/C20H22N4/c1-15(16-9-3-2-4-10-16)21-19-17-11-5-6-12-18(17)22-20(23-19)24-13-7-8-14-24/h2-6,9-12,15H,7-8,13-14H2,1H3,(H,21,22,23)
InChI key
HKGJEZIGDHFJFL-UHFFFAOYSA-N
Application
- to inhibit importin-β and to elucidate the mechanism of enhanced nuclear localization of heat shock protein 70 (HSP70) by vaccinia-related kinase 3 (VRK3)
- to treat COS?7 cells transfected with the expressing vectors of WT HNF4α , S78A, and S78D to study its effect
- as a KPNB inhibitor to determine whether CREB-regulated co-activator 1 (CRTC1) would be escorted into the nucleus by the classical nuclear localizing sequence (NLS)- dependent machinery
- as a functional inhibitor of Importin β in embryo treatment
- as an importin inhibitor to treat the transfected H1299 cells to block nuclear translocation of phosphorylated p53-RS
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service