T6074
Anti-α-Tubulin antibody, Mouse monoclonal

clone B-5-1-2, purified from hybridoma cell culture
Synonym(s):
Monoclonal Anti-α-Tubulin antibody produced in mouse, alpha-Tubulin
About This Item
Recommended Products
biological source
mouse
Quality Level
conjugate
unconjugated
antibody form
purified from hybridoma cell culture
antibody product type
primary antibodies
clone
B-5-1-2, monoclonal
form
buffered aqueous solution
mol wt
antigen ~50 kDa
species reactivity
mouse, chicken, Chlamydomonas, African green monkey, human, rat, bovine, sea urchin, kangaroo rat
enhanced validation
independent ( Antibodies)
Learn more about Antibody Enhanced Validation
concentration
~2 mg/mL
technique(s)
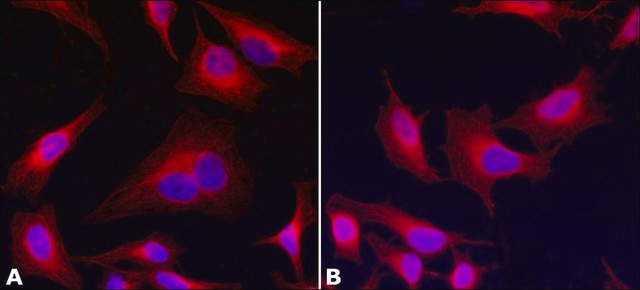
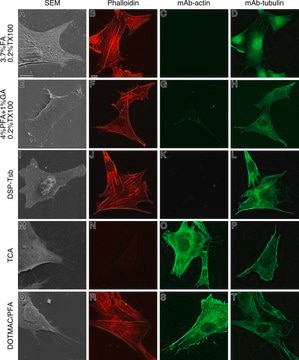
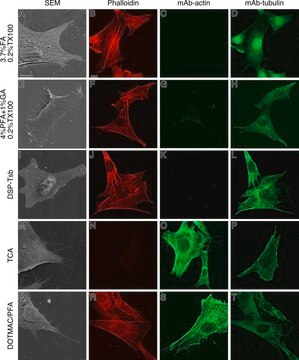
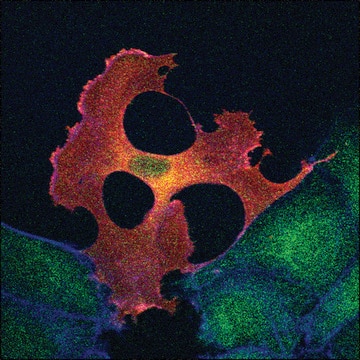
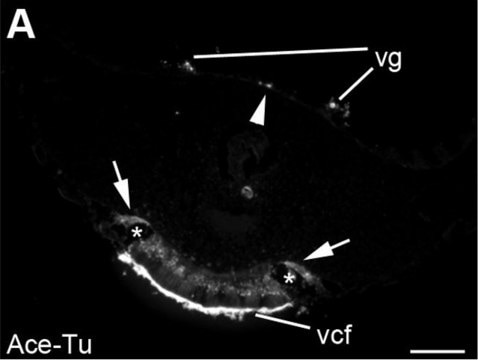
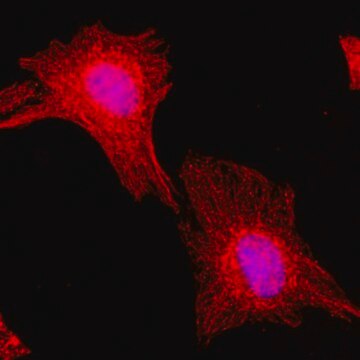
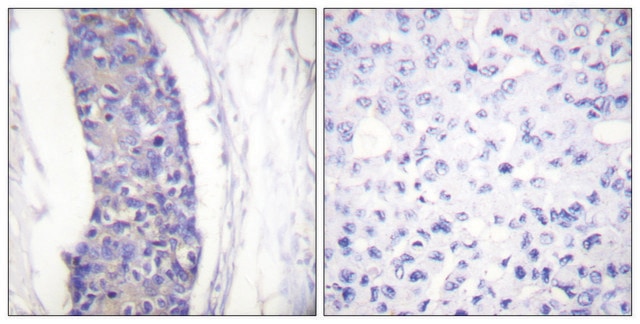
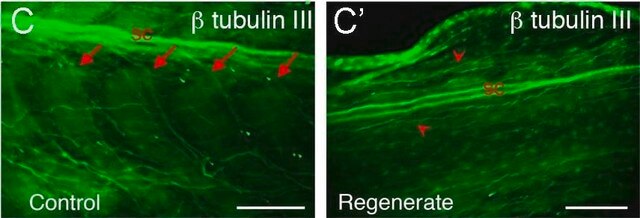
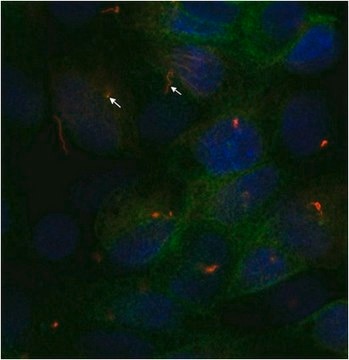
immunocytochemistry: 0.5-1 μg/mL using cultured chicken fibroblasts (CFB)
immunoprecipitation (IP): suitable
microarray: suitable
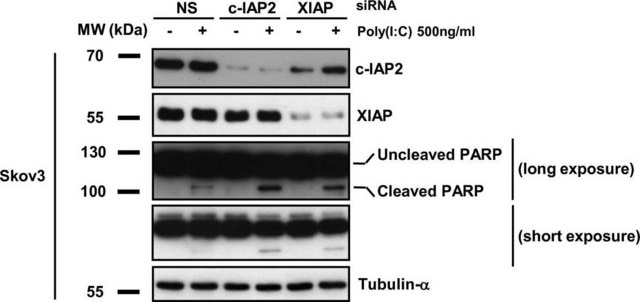
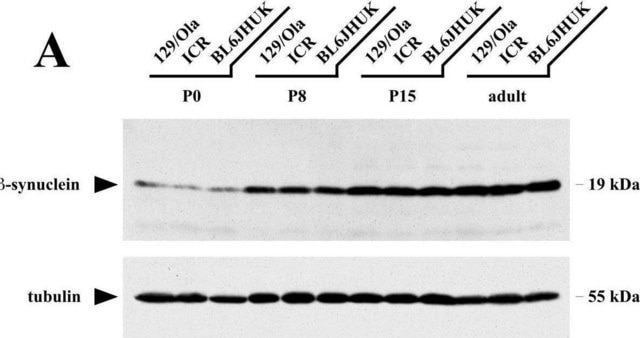
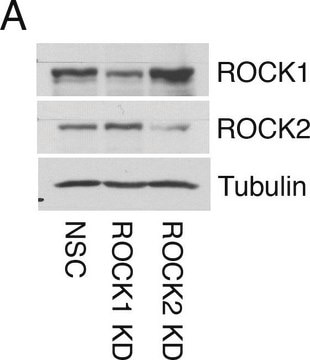
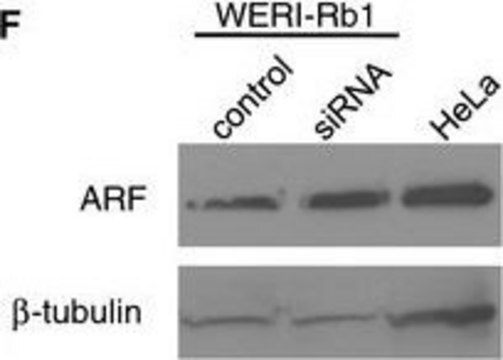
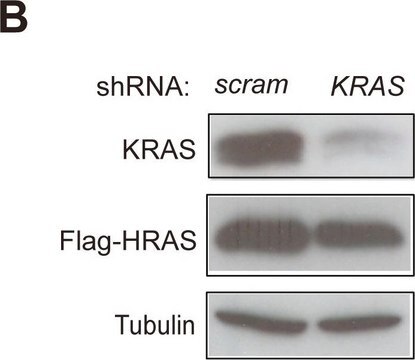
western blot: 0.25-0.5 μg/mL using total cell extract of human foreskin fibroblast cell line (FS11)
isotype
IgG1
UniProt accession no.
application(s)
research pathology
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... TUBA4A(7277)
mouse ... Tuba1a(22142)
rat ... Tuba1a(64158)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Monoclonal Anti-α-Tubulin (mouse IgG1 isotype) is derived from the hybridoma B-5-1-2 produced by the fusion of mouse myeloma cells and splenocytes from mice immunized with Sarkosyl-resistant filaments from Strongylocentrotus purpuratus (sea urchin).
Tubulin is a heterodimer that consists of α-tubulin and β-tubulin. Both subunits have a molecular weight of approx. 50 kDa and share considerable homology. In addition to α- and β-tubulin, several other tubulins have been identified, bringing the number of distinct tubulin classes to seven. Most of these tubulins have distinct subcellular localization and an emerging diverse set of functions. Tubulin is the major building block of microtubules. This intracellular, cylindrical, filamentous structure is present in almost all eukaryotic cells. Microtubules function as structural and mobile elements in mitosis, intracellular transport, flagellar movement, and the cytoskeleton.
Microtubular systems contain at least three α-tubulin isoforms. Two isoforms are coded by two αt-ubulin genes, which are both transcribed and code for extremely similar proteins. The third isoform is generated by post-translational modification.
Specificity
Immunogen
Application
Biochem/physiol Actions
α-Tubulin is a key regulator of cytoskeletal proteins. It mediates cellular developmental stages such as proliferation, migration, signalling and also maintains the shape of the cell. α-Tubulin controls trafficking, signaling and cellular tensegrity mediated by microtubules. The encoded protein is associated with the development and progression of cancer. α-Tubulin acetylation potentiates the metastatic property of breast cancer. Mutation in TUBA4A is associated with the development of various types of cancers, such as oral cancer, breast cancer, rectal cancer, lung cancer and prostate cancer. In addition, variation in the TUBA4A leads to sporadic amyotrophic lateral sclerosis (ALS).
Microtubules function as structural and mobile elements in mitosis, intracellular transport, flagellar movement, and the cytoskeleton.
Physical form
Storage and Stability
Disclaimer
Still not finding the right product?
Give our Product Selector Tool a try.
Storage Class
12 - Non Combustible Liquids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service