PHR1296
Bifonazole
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
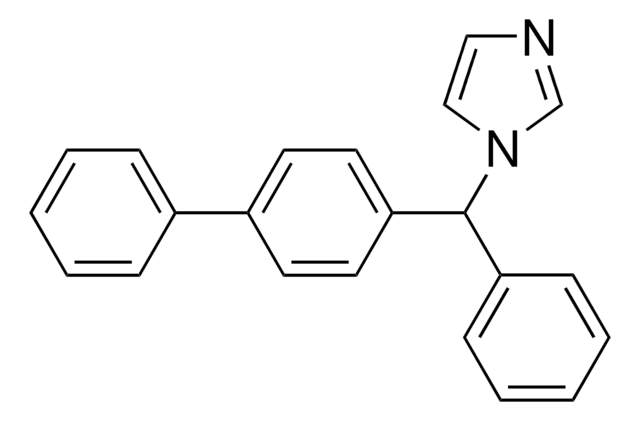
1-(p,α-Diphenylbenzyl)imidazole
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to Ph. Eur. B1110000
API family
bifonazole
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
SMILES string
c1ccc(cc1)C(c2ccc(cc2)-c3ccccc3)n4ccnc4
InChI
1S/C22H18N2/c1-3-7-18(8-4-1)19-11-13-21(14-12-19)22(24-16-15-23-17-24)20-9-5-2-6-10-20/h1-17,22H
InChI key
OCAPBUJLXMYKEJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Bifonazole is a substituted imidazole analog with therapeutic potential in treating invasive mucosal infections. It exhibits antifungal activity by suppressing the proliferation of dermatophytes, yeasts and fungi affecting the skin and nails. Its mode of action involves blocking the fungal ergosterol biosynthetic pathway and additional inhibition of terpenoid biosynthesis.
Application
Biochem/physiol Actions
Analysis Note
Other Notes
Footnote
related product
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service